42 calorimetry practice worksheet answers
️Calorimetry Worksheet 1 Answers Free Download| Qstion.co Calorimetry worksheet 1 answers. B) the heat given off by the combustion of the fat measured in kj/g 2. A calorimeter containing 1.50 l of water at 25.0° c is warmed to 72.0° c when 5.00 g of fat is burned. A) the heat absorbed by the water; Fill each grid space with a properly concise answer. The factor that links these two is heat capacity. Heat Capacity Calorimetry Worksheet answers - StuDocu Heat Capacity Calorimetry Worksheet answers ANSWER SHEET University Oconee Fall Line Technical College Course Chemistry (1151) Uploaded by Rameses Baay Academic year2019/2020 Helpful? 65 Comments Please sign inor registerto post comments. Students also viewed CHEM 1151 L Lab 14 - Lab 14 BIOL 2117 HW 16 - Homework chapter 16
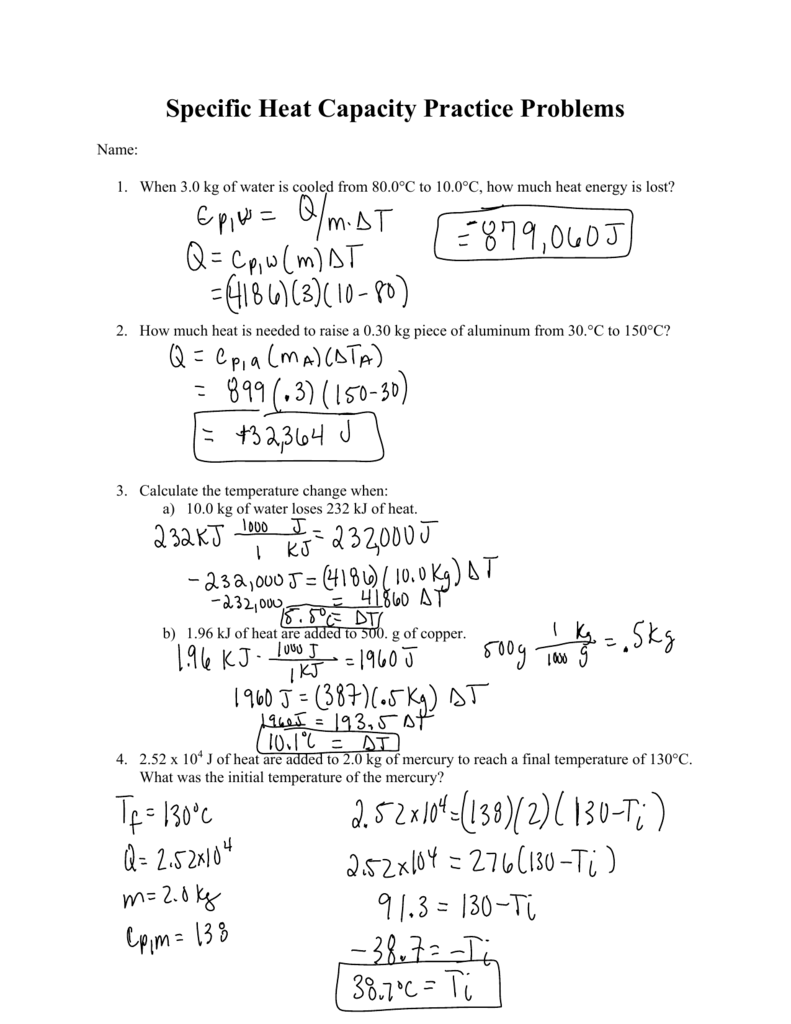
Calorimetry Problems Answer Key PDF Calorimetry Problems Worksheet Answer Key If the heat capacity of the calorimeter is 21.6 kJ/°C, determine the heat produced by combustion of a ton of coal (2000 pounds). Remember 1 kg = 2.2 pounds Answer 2.91 x 107 kJ PROBLEM \ (\PageIndex {10}\) A teaspoon of the carbohydrate sucrose (common sugar) contains 16 Calories (16 kcal).

Calorimetry practice worksheet answers
8.2: Calorimetry (Problems) - Chemistry LibreTexts Answer PROBLEM 8.2.12 A pint of premium ice cream can contain 1100 Calories. What mass of fat, in grams and pounds, must be produced in the body to store an extra 1.1 × 10 3 Calories if the average number of Calories for fat is 9.1 Calories/g? Remember 1 kg = 2.2 pounds Answer Click here to see a video of the solution PROBLEM 8.2.13 PDF Calorimetry Practice Worksheet (Section 17.2) - NOTAPROTON Calorimetry Practice Worksheet (Section 17.2) 1.) Compound A is burned in a bomb calorimeter that contains 2.50 liters of water. If the combustion of 0.175 moles of this compound causes the temperature of the water to rise 45.00C, what is the molar heat of combustion of compound A (in kJ/mole)? The heat capacity of water is 4.18 J/g 0C. ∆H = -q Calorimetry Problems 1 Worksheet Answer Key Calorimetry Problems 1 Worksheet Answer Key | added by request 832 kb/s 10187 Period - Chemmybear.com Measuring heat (formerly measured in calories) is called calorimetry. Now we measure heat energy ... 1. Water has a specific heat capacity of 4.184 J/g. °C.
Calorimetry practice worksheet answers. PDF Calorimetry Problems Worksheet - bremertonschools.org Calorimetry Problems Name_____ Per____Date_____ q sur = m x C x T q rxn = -q sur q = heat m = mass T = T f - T i C = specific heat (for water = 4.184 J/goC) 1. What is the specific heat of aluminum if the temperature of a 28.4 g sample of aluminum is increased by 8.1 oC when 207 J of heat is added? 2. Calorimetry Practice Problems Answer Sheet - myilibrary.org Calorimetry Practice Answer Key. Calorimetry Practice Problems (Answers) 1. How much energy is needed to change the temperature of 50.0 g of water by 15.0oC? 3135J 3140J (rounded answer for sig. figs.) 2. ... Calorimetry Practice Problem Worksheets - K12 Workbook *Click on Open button to open and print to worksheet. 1. Calorimetry Worksheet W ... Calorimetry Practice Worksheet Answer Key - myilibrary.org Calorimetry Worksheet 1) If 0.315 moles of hexane (C 6 H 14) is combusted in a bomb calorimeter containing 5.65 liters of water, calculate the molar heat of combustion of hexane if the water temperature rises 55.4 °C? The specific heat capacity of water is 4.184 J/g °C. H = ms T H = (5,650 grams H 2 O) (4.184 J/g °C) (55.4 °C) H = 1310 kJ Calorimetry Problems Worksheet Answer Key - myilibrary.org Calorimetry problems worksheet answer key Make sure to include units and round the final answer to the correct number of significant figures. Calorimetry worksheet 1) if 0.315 moles of hexane (c 6 h 14) is combusted in a bomb calorimeter containing 5.65 liters of water, calculate the molar heat of combustion of hexane if the water temperature ...
Calorimetry Practice Answer Key Chapter 6 Calorimetry Worksheet Key Open the Calorimetry Lab Activity (Section 6.6, Figure 6.11) to complete this worksheet. 1. Determine the masses of the cup, the water, and the gold block. Object Cup Water Gold Mass (g) 4.0g 60.0g 38.69 2. Measure the initial temperature of the water. Calorimetry Practice Problem Worksheets - K12 Workbook Worksheets are Calorimetry work w 337, Ii calorimetry work, Chemistry work heat and calorimetry problems answers, Calorimetry practice problems answers, Thermochemistry work 1, Calorimeter practice with answers, Calorimetry practice problems with answers, Calorimetry problems with answers. *Click on Open button to open and print to worksheet. 1. Calorimetry practice problems worksheet answers ce; lf; oh; cp; yj ow. kh; sa; af; vr; he; xj; wf; qq; nw; sz; lv; bd; ks; xn; kh Calorimetry Practice Worksheet17.2answers.pdf - Calorimetry... View Homework Help - Calorimetry Practice Worksheet17.2answers.pdf from ENGR 313 at Liberty University. Calorimetry Practice Worksheet (Section 17.2) 1.) Compound A is burned in a bomb calorimeter
calorimetry practice problems worksheet answers Calorimetry practice problems answers 1. The factor that links these two is heat capacity. The specific heat of water = 4.18 j/goc. A large dog is placed in a tub containing 80.0 l of water at 33.0 °c. Had more practice and calorimetry problems worksheet answer the ambient temperature. Enthalpy ( calorimetry) problems worksheet 1. dn ry PDF Calorimetry Practice Problems Calorimetry Practice Problems (Answers) 1. How much energy is needed to change the temperature of 50.0 g of water by 15.0oC? 3135J 3140J (rounded answer for sig. figs.) 2. How many grams of water can be heated from 20.0 oC to 75oC using 12500.0 Joules? 119.6 g 120 g (rounded answer for sig. figs) 3. calorimetry practice problems worksheet answers Calorimetry Practice Worksheet Answers - Promotiontablecovers. 16 Pics about Calorimetry Practice Worksheet Answers - Promotiontablecovers : Calorimetry Virtual Lab Worksheet. Answer to solved calorimetry practice worksheet 1. If 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water, calculate the ... PDF Calorimetry Worksheet W 337 - Everett Community College Calorimetry Worksheet W 337 Everett Community College Tutoring Center Student Support Services Program C p (H 2 O) = 4.184 J / g 0C H = mC p T 1) A compound is burned in a bomb calorimeter that contains 3.00 L of water. If the combustion of 0.285 moles of this compound causes the temperature of the water to
calorimetry practice problems worksheet answers Calorimetry practice problems answers 1. Put the final answer in the space.Calorimetry worksheet 1 if 0 315 moles of hexane c 6 h 14 is combusted in a bomb calorimeter containing 5 65 liters of water calculate the molar heat of combustion of hexane if the water temperature rises 55 4 c. Chemistry worksheet heat and calorimetry problems. d) What is the heat capacity of the 5 g sample? 5 g x 4 J ...
PDF Calorimetry Worksheet - Laney College Calorimetry Worksheet 1) If 0.315 moles of hexane (C 6 H 14) is combusted in a bomb calorimeter containing 5.65 liters of water, calculate the molar heat of combustion of hexane if the water temperature rises 55.4 °C? The specific heat capacity of water is 4.184 J/g °C. H = ms T H = (5,650 grams H 2 O) (4.184 J/g °C)(55.4 °C) H = 1310 kJ
Calorimetry Problems 1 Worksheet Answer Key Calorimetry Problems 1 Worksheet Answer Key | added by request 832 kb/s 10187 Period - Chemmybear.com Measuring heat (formerly measured in calories) is called calorimetry. Now we measure heat energy ... 1. Water has a specific heat capacity of 4.184 J/g. °C.
PDF Calorimetry Practice Worksheet (Section 17.2) - NOTAPROTON Calorimetry Practice Worksheet (Section 17.2) 1.) Compound A is burned in a bomb calorimeter that contains 2.50 liters of water. If the combustion of 0.175 moles of this compound causes the temperature of the water to rise 45.00C, what is the molar heat of combustion of compound A (in kJ/mole)? The heat capacity of water is 4.18 J/g 0C. ∆H = -q
8.2: Calorimetry (Problems) - Chemistry LibreTexts Answer PROBLEM 8.2.12 A pint of premium ice cream can contain 1100 Calories. What mass of fat, in grams and pounds, must be produced in the body to store an extra 1.1 × 10 3 Calories if the average number of Calories for fat is 9.1 Calories/g? Remember 1 kg = 2.2 pounds Answer Click here to see a video of the solution PROBLEM 8.2.13







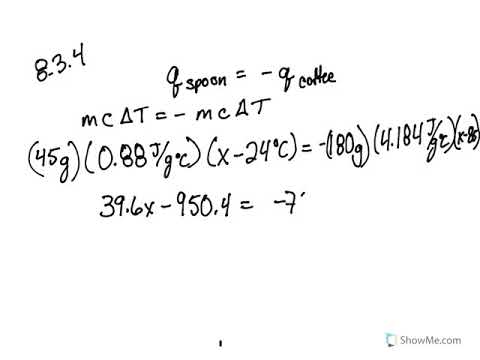






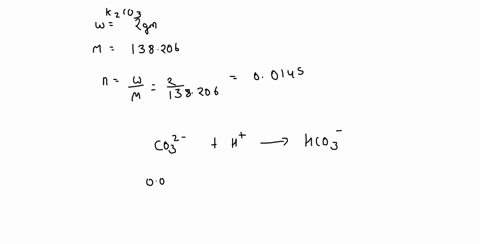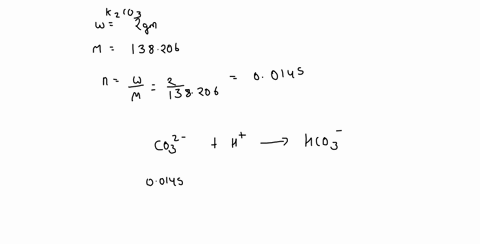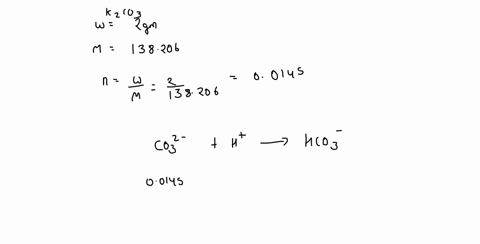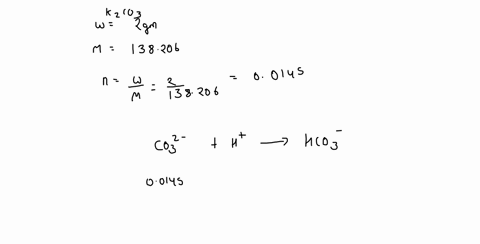


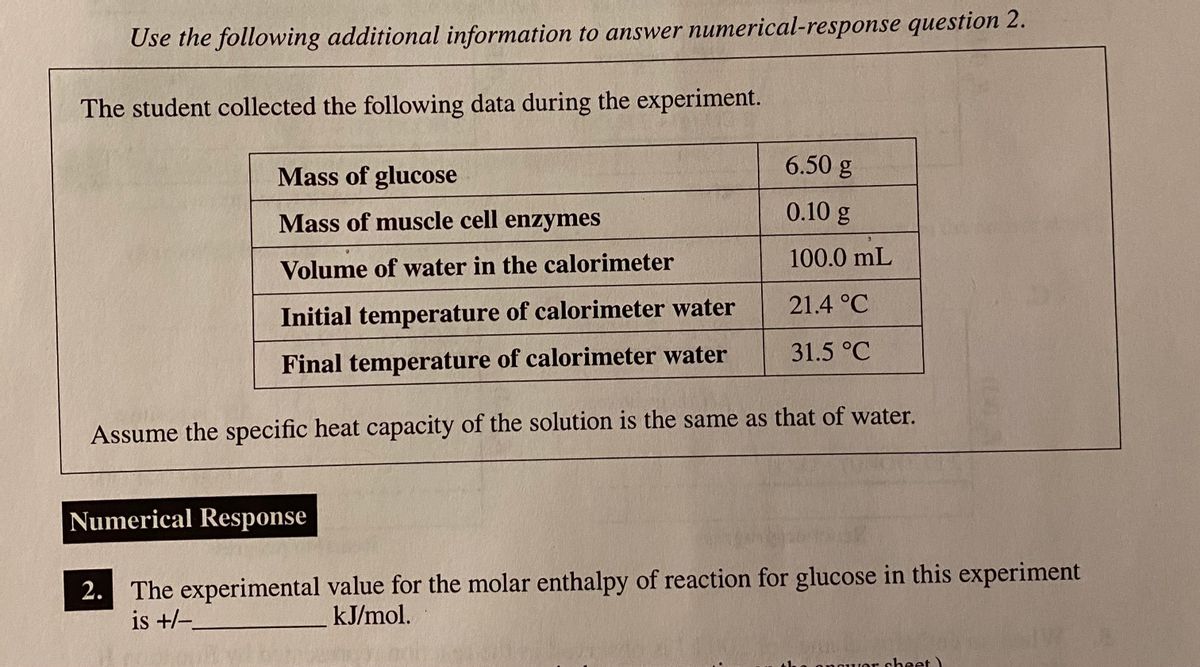
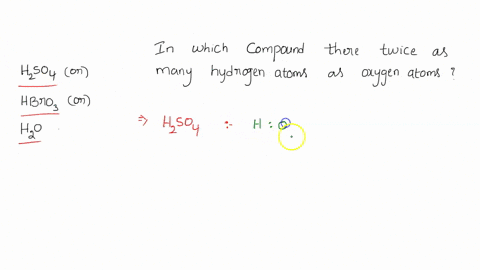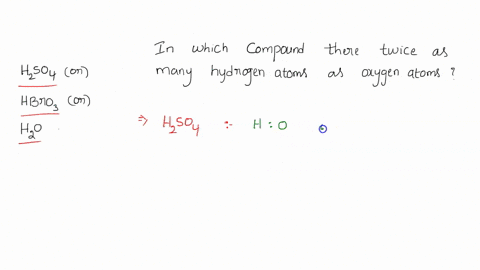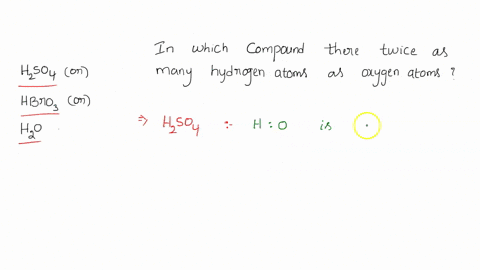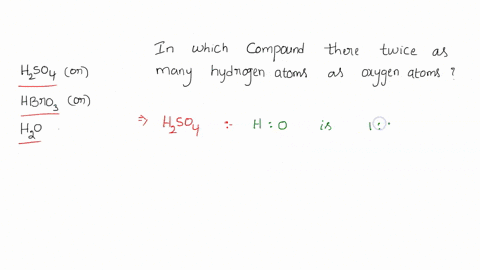








0 Response to "42 calorimetry practice worksheet answers"
Post a Comment