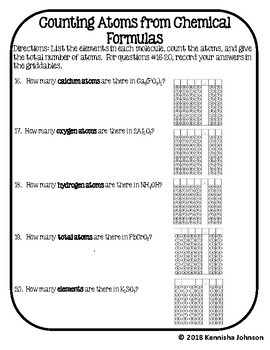
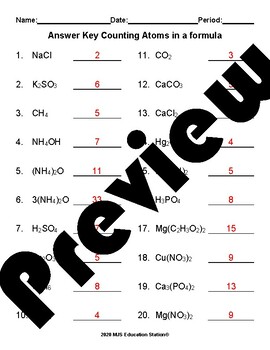
42 number of atoms in a formula worksheet answer key
eupolcopps.euThe EU Mission for the Support of Palestinian Police and Rule ... EUPOL COPPS (the EU Coordinating Office for Palestinian Police Support), mainly through these two sections, assists the Palestinian Authority in building its institutions, for a future Palestinian state, focused on security and justice sector reforms. This is effected under Palestinian ownership and in accordance with the best European and international standards. Ultimately the Mission’s ... › science › physicalAtom Worksheets Protons and neutrons have what is considered one atomic mass unit, but the electron has a lot less mass. In fact it has one-thousandths an atomic unit. Because of their opposite charges electrons and protons are attracted to one another holding the nucleus together somewhat. Because of the even charges, atoms are electrically neutral.
phet.colorado.edu › en › simulationMolecule Shapes - VSEPR | Lone Pairs | Bonds - PhET ... Explore molecule shapes by building molecules in 3D! How does molecule shape change with different numbers of bonds and electron pairs? Find out by adding single, double or triple bonds and lone pairs to the central atom. Then, compare the model to real molecules!
Number of atoms in a formula worksheet answer key
byjus.com › chemistry › mole-concept-basicsMole Concept- Formula, Explanations, Examples, Related ... The total number of atoms/molecules in a sample can be calculated by multiplying the number of moles with the Avogadro constant. This formula can be written as: Number of Atoms or Molecules = (Number of Moles)*(6.022*10 23) The relationship between the atomic mass unit (amu) and the gram is given by: 1 amu = (1gram)/(6.022*10 23) = 1.66*10-24 grams openstax.org › books › chemistry-2e4.1 Writing and Balancing Chemical Equations - OpenStax If an element appears in more than one formula on a given side of the equation, the number of atoms represented in each must be computed and then added together. For example, both product species in the example reaction, CO 2 and H 2 O, contain the element oxygen, and so the number of oxygen atoms on the product side of the equation is allinonehighschool.com › chemistry-with-lab-2018Chemistry with Lab – Easy Peasy All-in-One High School The key to this is to add up the number of electrons in the configuration (the raised numbers after the letters, which is the atomic number/number of electrons). For Quiz 3 and 4, count the number of electrons as before, then look the final number up as the atomic number on the periodic table where you will find the chemical symbol or name.
Number of atoms in a formula worksheet answer key. › classroomresourcesClassroom Resources - National Council of Teachers of Mathematics These stories and lesson sketches, focused in the middle and high school grades, are meant to help your students extend their view of the world a little bit by using math to make sense of experiences in daily life. allinonehighschool.com › chemistry-with-lab-2018Chemistry with Lab – Easy Peasy All-in-One High School The key to this is to add up the number of electrons in the configuration (the raised numbers after the letters, which is the atomic number/number of electrons). For Quiz 3 and 4, count the number of electrons as before, then look the final number up as the atomic number on the periodic table where you will find the chemical symbol or name. openstax.org › books › chemistry-2e4.1 Writing and Balancing Chemical Equations - OpenStax If an element appears in more than one formula on a given side of the equation, the number of atoms represented in each must be computed and then added together. For example, both product species in the example reaction, CO 2 and H 2 O, contain the element oxygen, and so the number of oxygen atoms on the product side of the equation is byjus.com › chemistry › mole-concept-basicsMole Concept- Formula, Explanations, Examples, Related ... The total number of atoms/molecules in a sample can be calculated by multiplying the number of moles with the Avogadro constant. This formula can be written as: Number of Atoms or Molecules = (Number of Moles)*(6.022*10 23) The relationship between the atomic mass unit (amu) and the gram is given by: 1 amu = (1gram)/(6.022*10 23) = 1.66*10-24 grams





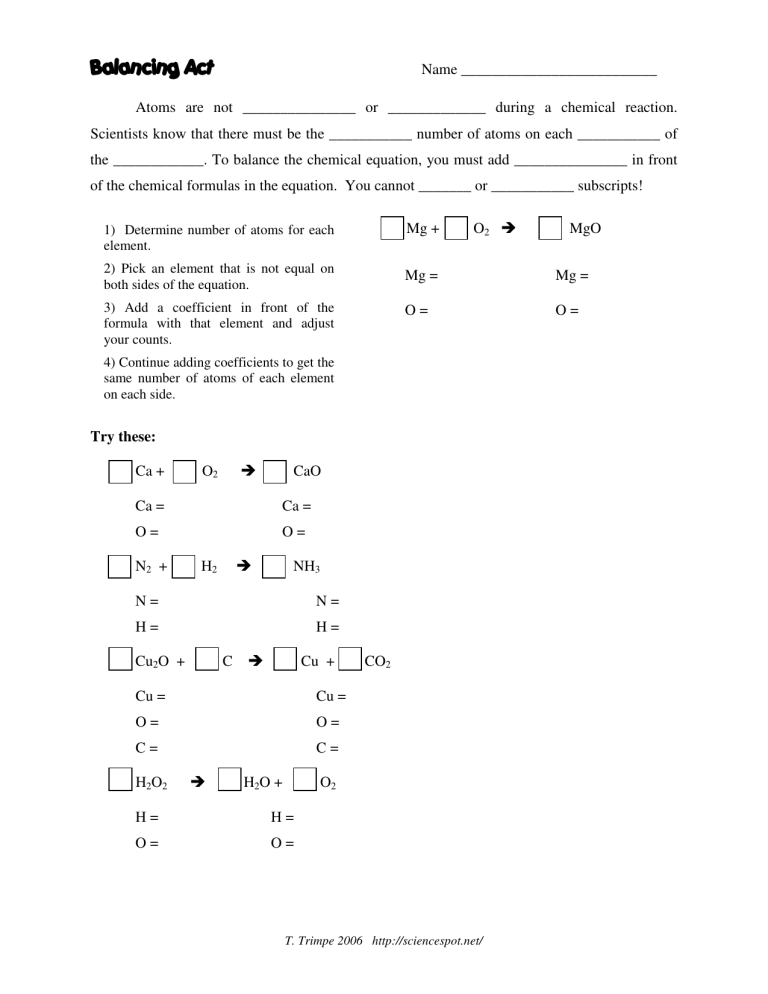
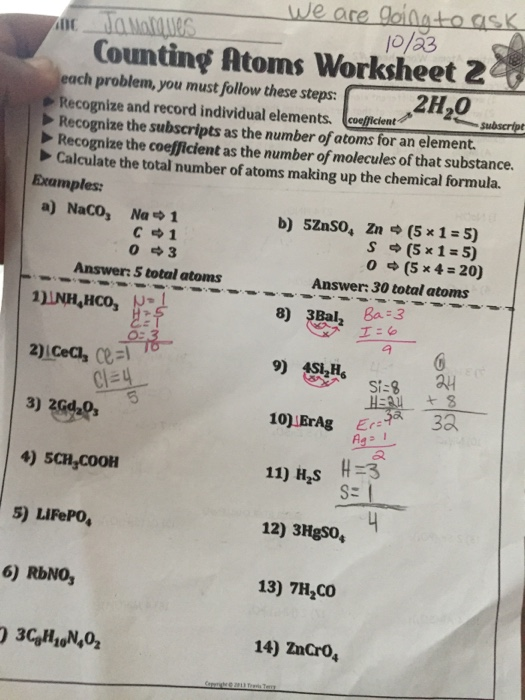







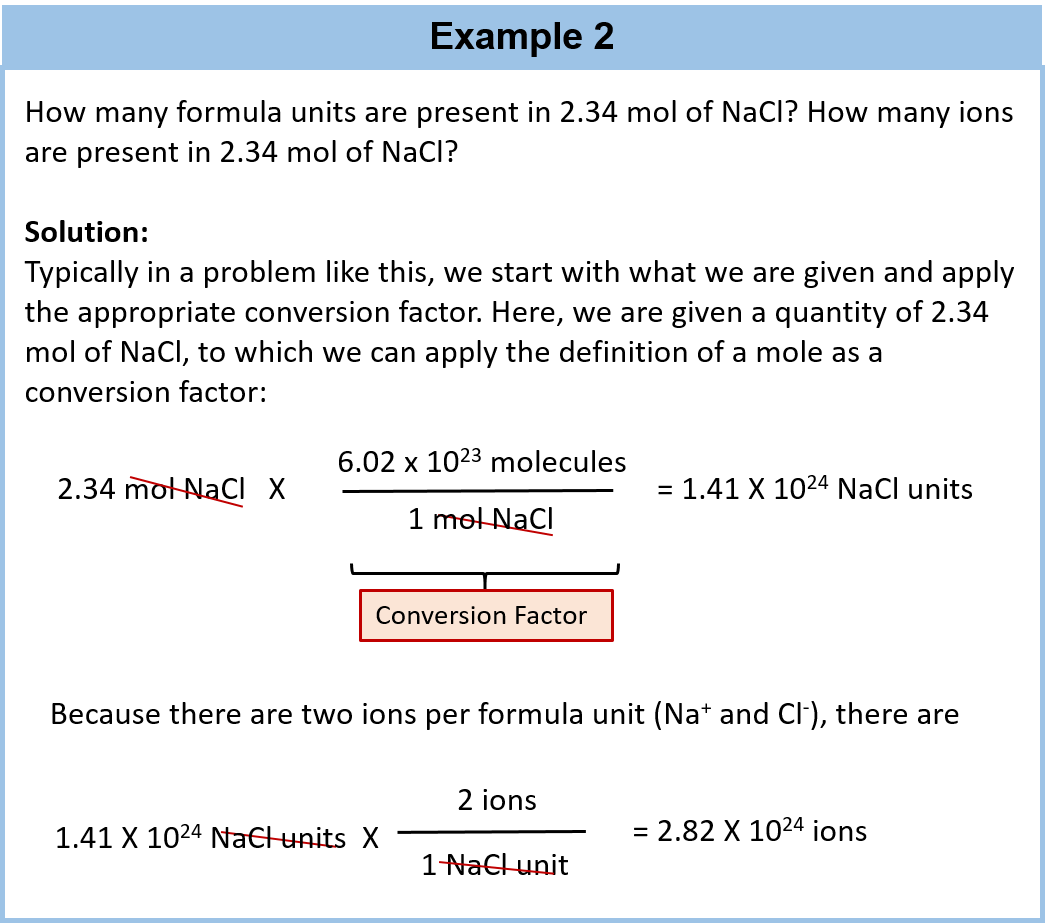


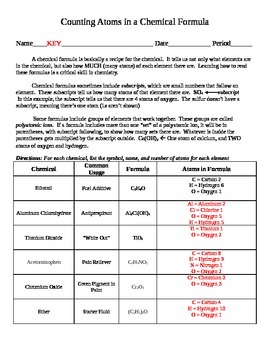







0 Response to "42 number of atoms in a formula worksheet answer key"
Post a Comment