41 chemical formulas and nomenclature worksheet
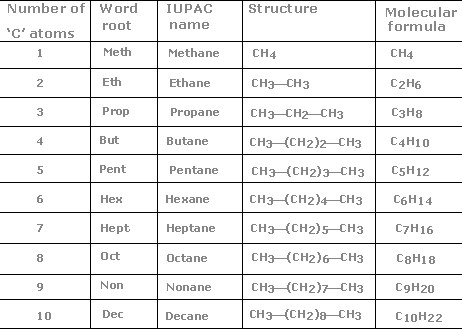
Chemistry Word Search Topics Chemistry word searches help students to remember complicated chemistry terms. Some word searches list common elements, and others list chemical reactions. Teachers created these chemistry word searches for their students, and you can, too. 4.1 Writing and Balancing Chemical Equations - OpenStax Extending this symbolism to represent both the identities and the relative quantities of substances undergoing a chemical (or physical) change involves writing and balancing a chemical equation. Consider as an example the reaction between one methane molecule (CH 4 ) and two diatomic oxygen molecules (O 2 ) to produce one carbon dioxide ...
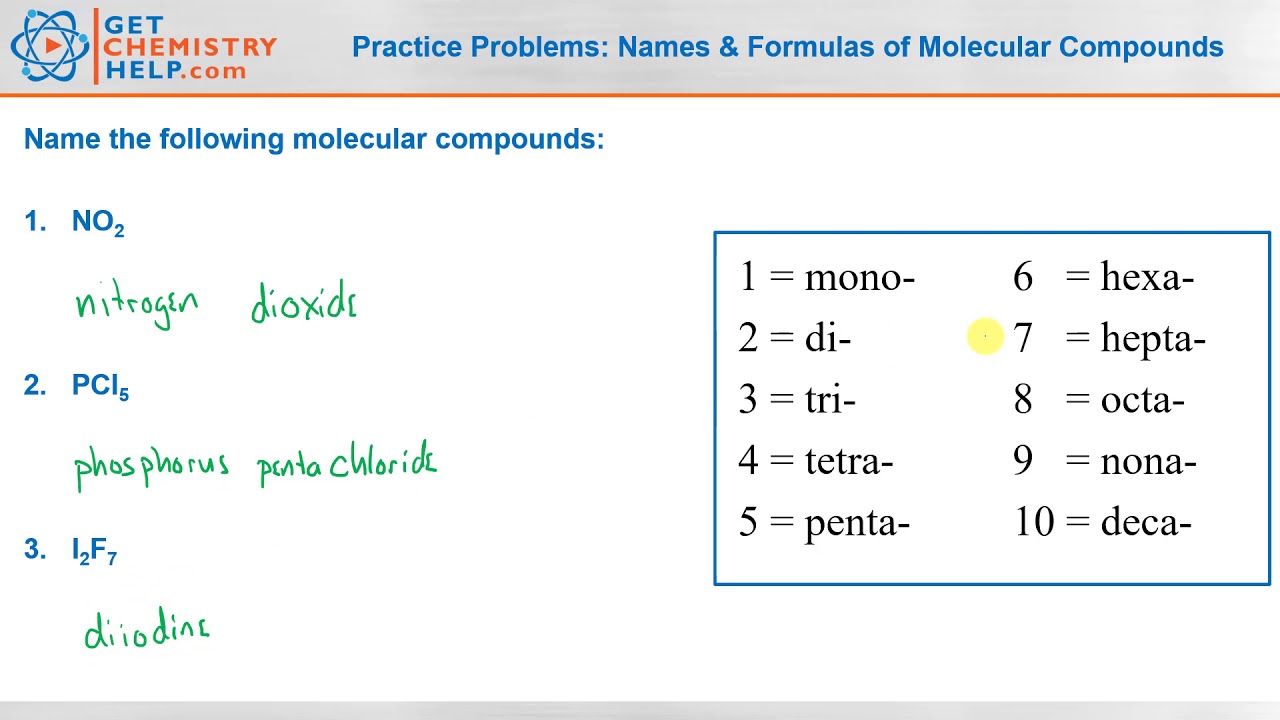
How to Write a Chemical Equation (with Pictures) - wikiHow Oct 07, 2022 · If you want to write a chemical equation, start by writing the chemical formulas of each reactant. Use the prefixes, such as mono-, di-, tri-, and tetra-, to figure out the number of atoms present for each element, and write this number as a subscript for each element. For example, dihydrogen monoxide would be more easily written as H2O.
Chemical formulas and nomenclature worksheet
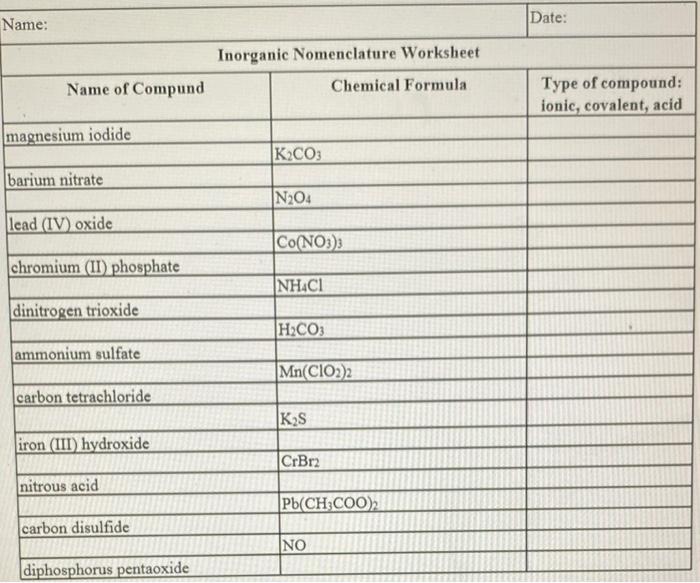
5.5: Writing Formulas for Ionic Compounds - Chemistry LibreTexts Sep 25, 2022 · The rule for constructing formulas for ionic compounds containing polyatomic ions is the same as for formulas containing monatomic (single-atom) ions: the positive and negative charges must balance. If more than one of a particular polyatomic ion is needed to balance the charge, the entire formula for the polyatomic ion must be enclosed in ... 2.3: Families and Periods of the Periodic Table Since the families of elements were organized by their chemical behavior, it is predictable that the individual members of each chemical family will have similar electron configurations. Families of the Periodic Table. Remember that Mendeleev arranged the periodic table so that elements with the most similar properties were placed in the same group. A group is a vertical column of the … Naming Ionic Compounds Practice Worksheet Review– Naming Chemical Compounds The following are a good mix of naming and formula writing problems to help you get some practice. Name the following chemical compounds: 1) NaBr _____ 2) Ca(C 2 H 3 O 2) 2 _____ 3) P 2 O 5 _____
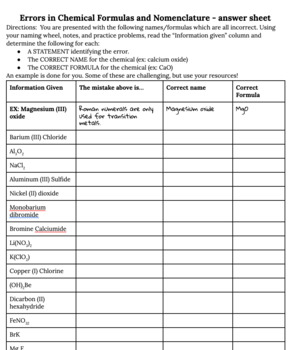
Chemical formulas and nomenclature worksheet. CSUS Chemistry 1A Nomenclature Worksheet Dr. Mack CSUS Chemistry 1A Nomenclature Worksheet Dr. Mack Page 5 of 9 Nomenclature of Ionic and Covalent Compounds 1. Binary Ionic Compounds Containing a Metal and a Nonmetal. A binary compound is a compound formed from two different elements. There may or may not be more than one of each element. Writing Ionic Formulas: Introduction - YouTube Here's how to write formulas for binary ionic compounds. We'll see how you have to balance the charges of the two ions so they cancel each other out. Naming Ionic Compounds Practice Worksheet Review– Naming Chemical Compounds The following are a good mix of naming and formula writing problems to help you get some practice. Name the following chemical compounds: 1) NaBr _____ 2) Ca(C 2 H 3 O 2) 2 _____ 3) P 2 O 5 _____ 2.3: Families and Periods of the Periodic Table Since the families of elements were organized by their chemical behavior, it is predictable that the individual members of each chemical family will have similar electron configurations. Families of the Periodic Table. Remember that Mendeleev arranged the periodic table so that elements with the most similar properties were placed in the same group. A group is a vertical column of the …
5.5: Writing Formulas for Ionic Compounds - Chemistry LibreTexts Sep 25, 2022 · The rule for constructing formulas for ionic compounds containing polyatomic ions is the same as for formulas containing monatomic (single-atom) ions: the positive and negative charges must balance. If more than one of a particular polyatomic ion is needed to balance the charge, the entire formula for the polyatomic ion must be enclosed in ...




























0 Response to "41 chemical formulas and nomenclature worksheet"
Post a Comment