43 protons neutrons and electrons practice worksheet
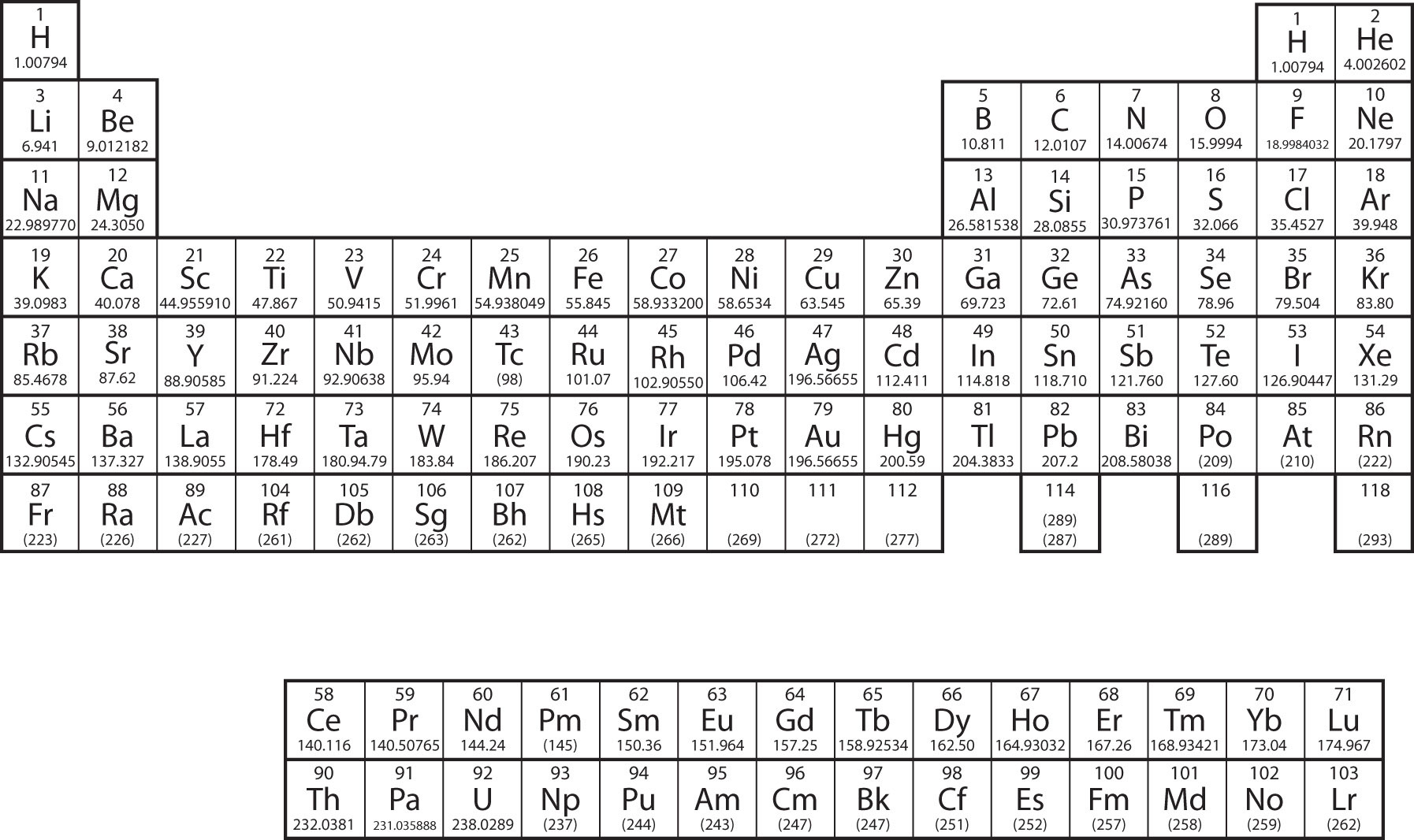
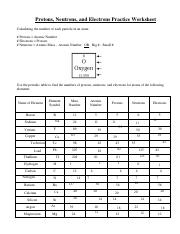
Protons, Neutrons, and Electrons Practice Worksheet Protons, Neutrons, and Electrons Practice Worksheet. Atomic. symbol. Atomic. number. Protons, Neutrons, Electrons, Mass. Protons, Neutrons, and Electrons Practice Worksheet Protons, Neutrons, and Electrons Practice Worksheet Calculating the number of each particle in an atom: # Protons = Atomic Number # Electrons = Protons # Neutrons = Atomic Mass – Atomic Number OR Big # - Small # Use the periodic table to find the numbers of protons, neutrons, and electrons for atoms of the following elements. Name of Element
Periodic Table Worksheet & Explanation - 7th Grade Science ... That is number of protons= number of electrons orbiting the nucleus of an atom = atomic number. The atomic mass is the average mass of the protons, electrons and neutrons in a single atom. An atom of carbon with protons and neutrons (nucleus) in the …

Protons neutrons and electrons practice worksheet
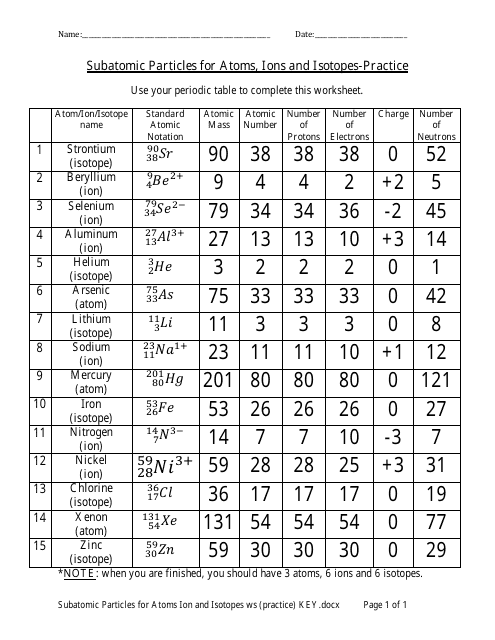
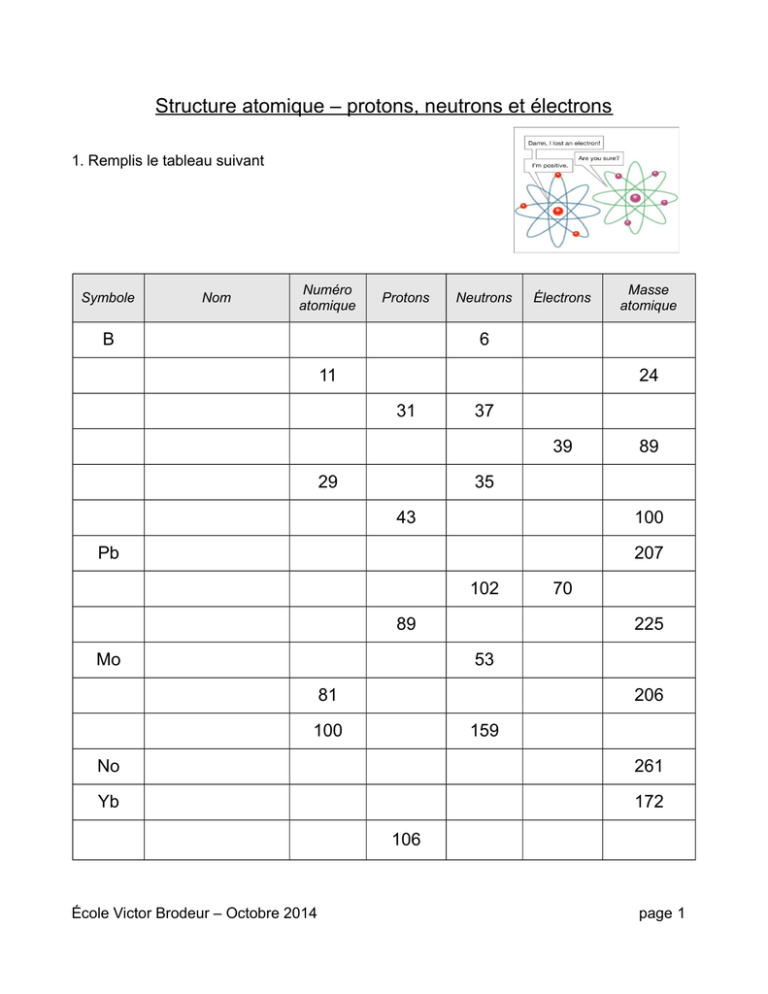
Protons, Neutrons, and Electrons Practice Worksheet - Google Docs Protons, Neutrons, and Electrons Practice Worksheet - Google Docs Protons, Neutrons, and Electrons Practice Worksheet Helpful Concepts: # Protons + # Neutrons = Atomic mass # (this is usually shown... Protons, Neutrons, and Electrons Practice Worksheet Protons, Neutrons, and Electrons Practice Worksheet Fill in the blanks in the following worksheet. Please keep in mind that the isotope represented by each space may NOT be the most common isotope or the one closest in atomic mass to the value on the periodic table. Atomic symbol Atomic number Protons Neutrons Electrons Atomic mass B 6 What Is a Subatomic Particle? - Definition & Mass - Video ... 17.9.2021 · Electrons are very small in mass when compared to protons and neutrons. It takes more than 1,800 electrons to equal the mass of one proton. In fact, the mass of an electron is so small that it is ...
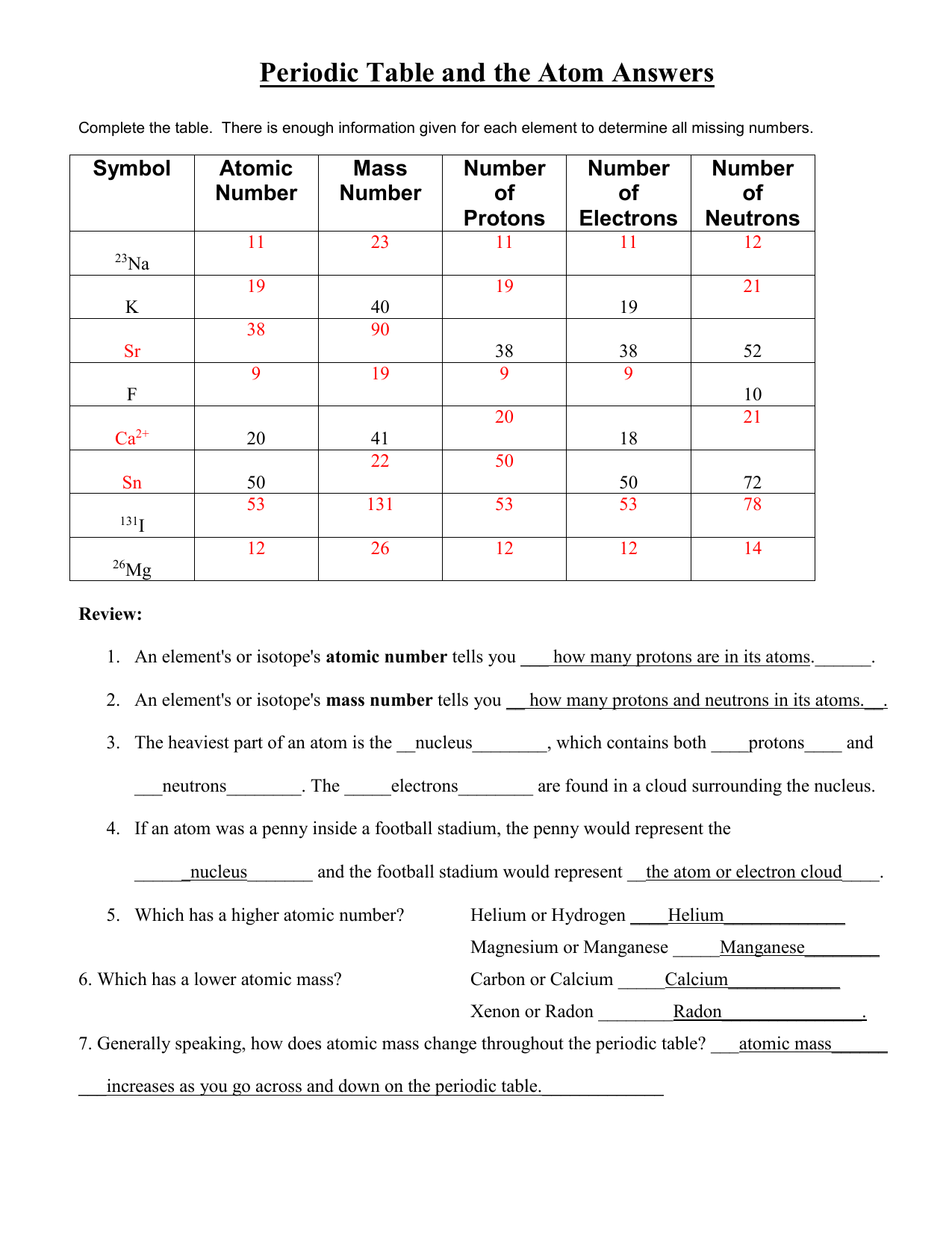
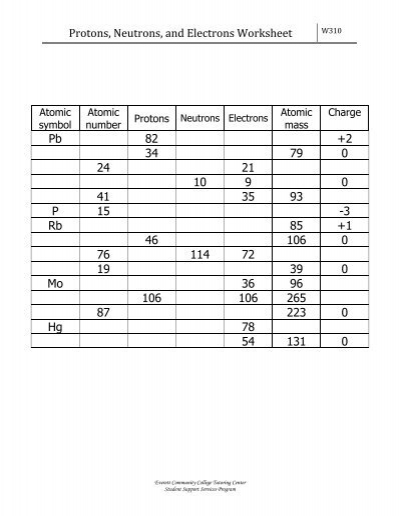
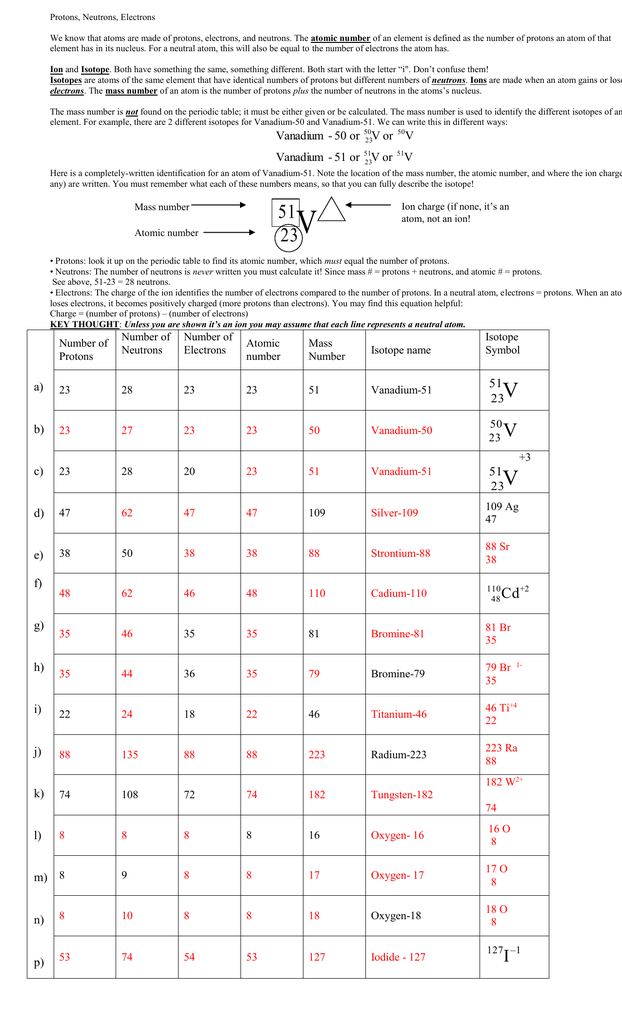
Protons neutrons and electrons practice worksheet. Isotope Practice Worksheet - Chemistry Isotope Practice 1. Here are three isotopes of an element: 12C 14 13C C a. The element is: Carbon b. The number 6 refers to the Atomic Number c. The numbers 12, 13, and 14 refer to the Mass Number d. How many protons and neutrons are in the first isotope? 6 protons & 6 neutrons e. How many protons and neutrons are in the second isotope? The Structure of Matter - Physics Classroom An understanding of how objects becomes charged begins with an understanding of the structure of the atom. The atom consists of uncharged neutrons and positively-charged protons densely packed into the center of the atom - known as the nucleus. Surrounding the nucleus are negatively-charged electrons that are located in regions of space known as electron shells. Atomic Neutrons Electrons Atomic Charge Protons mass Protons, Neutrons, and Electrons Worksheet W310 Everett Community College Tutoring Center Student Support Services Program Atomic symbol Atomic number Protons Neutrons Electrons Atomic mass Charge Pb 82 +2 34 79 0 24 21 10 9 0 41 35 93 P 15 -3 Rb 85 +1 46 106 0 76 114 72 Protons, Neutrons, and Electrons Practice Worksheet Protons, Neutrons, and Electrons Practice Worksheet Calculating the number of each particle in an atom: # Protons = Atomic Number # Electrons = Protons # Neutrons = Atomic Mass – Atomic Number OR Big # - Small # Use the periodic table to find the numbers of protons, neutrons, and electrons for atoms of the following elements.
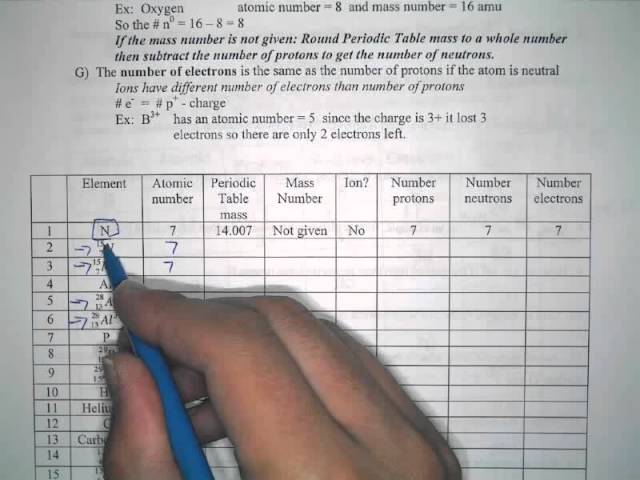
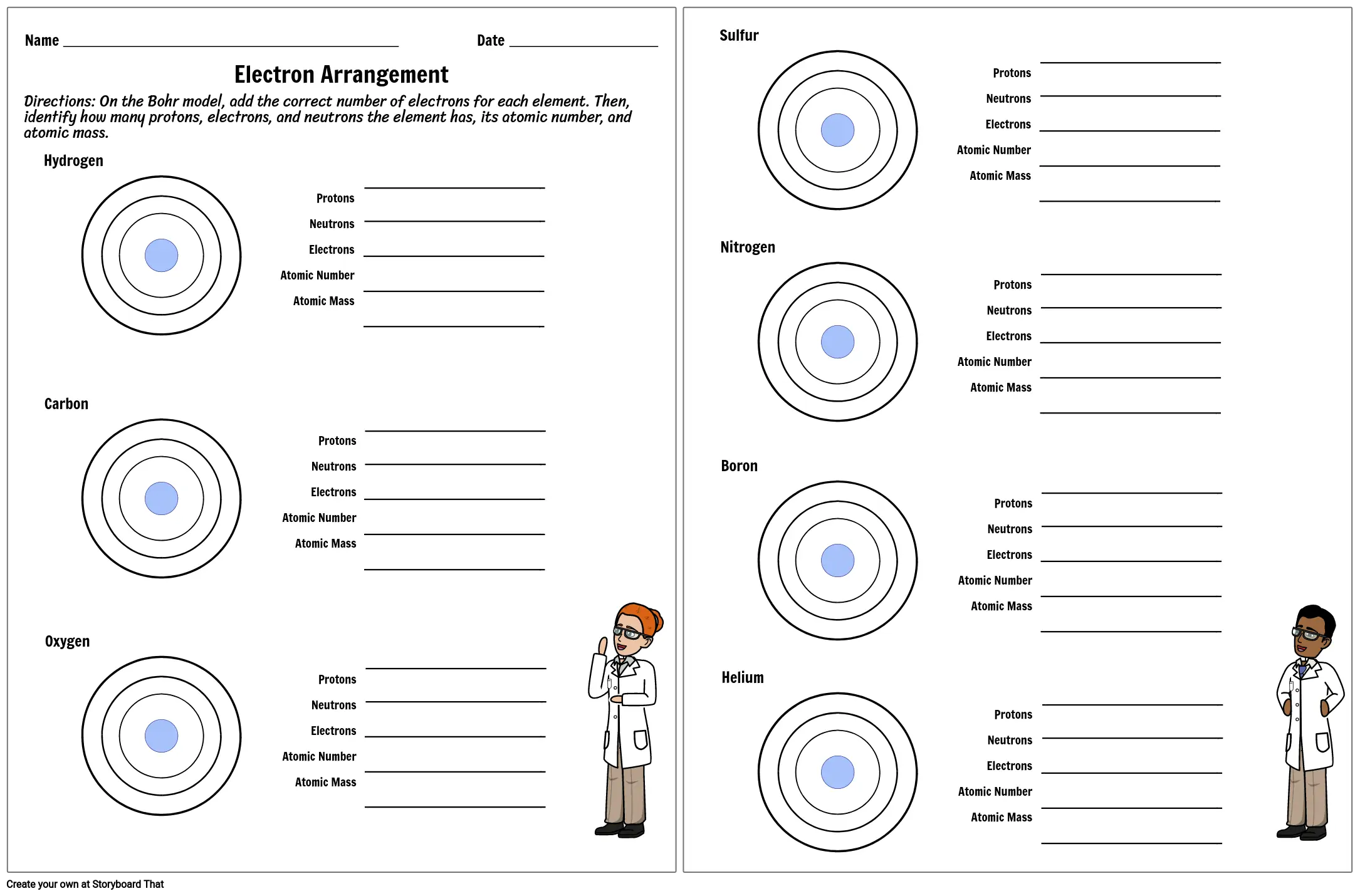
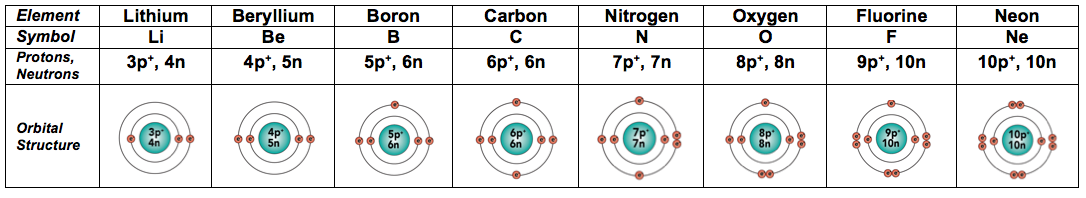
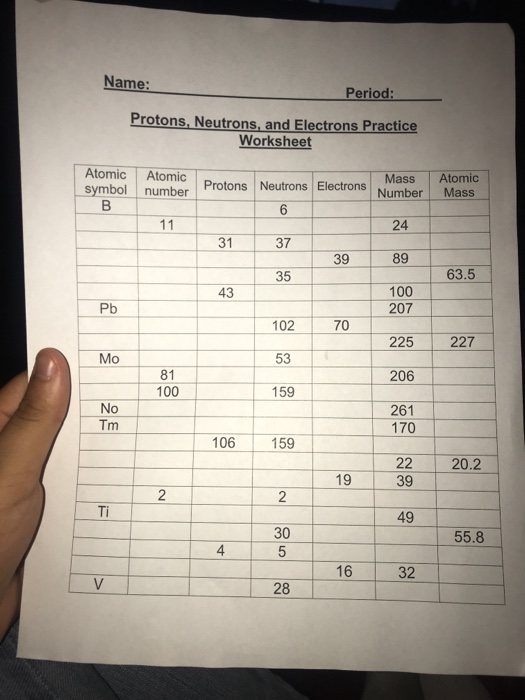
Protons, Neutrons, and Electrons Practice Worksheet Protons, Neutrons, and Electrons Practice Worksheet Fill in the blanks in the following worksheet. Please keep in mind that the isotope represented by each space may NOT be the most common isotope or the one closest in atomic mass to the value on the periodic table. Atomic symbol Atomic number ProtonsNeutrons Electrons Atomic mass Protons, Neutrons, and Electrons Practice Worksheet - Obion ... Protons, Neutrons, and Electrons Practice Worksheet. How to calculate the number of each particle in an atom: # Protons = Atomic Number. # Neutrons = Atomic ... Isotope Worksheet Answer Key - ISD 622 Isotope Practice Worksheet Name: 1. 2. D. 4. 13 12 Here are three isotopes of an element: a. The element is: b. The number 6 refers to the c. The numbers 12, 13, and 14 refer to the d. How many protons and neutrons are in the first isotope? (O e. How many protons and neutrons are in the second isotope? f. Atomic Protons Neutrons Electrons Lewis Dot Mass Lewis Dot Diagram Worksheet Use the Bohr models to determine the number of valance electrons. Once you have found the number of valance electrons, place them around the elements symbol. Element Atomic # Atomic Mass Protons Neutrons Electrons Lewis Dot Carbon 6 12 6 6 6 l Hydrogen 1 1 1 0 1 H Lithium 3 7 3 4 3 Li
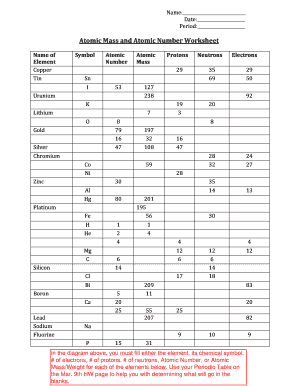
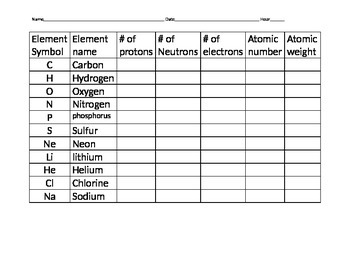
Atomic Mass and Atomic Number Worksheet Key Atomic Mass and Atomic Number Worksheet - Key Name of Element Symbol Atomic Number Atomic Mass Protons Neutrons Electrons copper Cu 29 64 29 35 29 tin Sn 50 119 50 69 50 iodine I 53 127 53 74 53 uranium U 92 238 92 146 92 potassium K 19 39 19 20 19 lithium Li 3 7 3 4 3 oxygen O 8 16 8 8 8 gold Au 79 ... What Is a Subatomic Particle? - Definition & Mass - Video ... 17.9.2021 · Electrons are very small in mass when compared to protons and neutrons. It takes more than 1,800 electrons to equal the mass of one proton. In fact, the mass of an electron is so small that it is ... Protons, Neutrons, and Electrons Practice Worksheet Protons, Neutrons, and Electrons Practice Worksheet Fill in the blanks in the following worksheet. Please keep in mind that the isotope represented by each space may NOT be the most common isotope or the one closest in atomic mass to the value on the periodic table. Atomic symbol Atomic number Protons Neutrons Electrons Atomic mass B 6 Protons, Neutrons, and Electrons Practice Worksheet - Google Docs Protons, Neutrons, and Electrons Practice Worksheet - Google Docs Protons, Neutrons, and Electrons Practice Worksheet Helpful Concepts: # Protons + # Neutrons = Atomic mass # (this is usually shown...

![Protons Neutrons And Electrons Practice Ws [6nq85590eznw]](https://idoc.pub/img/crop/300x300/6nq85590eznw.jpg)


















0 Response to "43 protons neutrons and electrons practice worksheet"
Post a Comment