42 ph of salt solutions worksheet answers
Bottom text Laboratory Exercise 3 pH of solutions. Hydrolysis of salts. English version written by dr Hydrolysis of salts Hydrolysis reaction is the reverse reaction to neutralization reaction, salts react Answer: pH=10,63 9. Write a hydrolysis reaction of BaCO3 and state whether a salt solution is acidic, basic or.
Hello! My professor said that we should prepare our answers or solutions in a worksheet. How should I do it (cash and cash equivalents) in a worksheet? Thank you. Problem 1-10 (AICPA Adapted) Tranvia Company revealed the following information on December 31, 2019: 350,000 750,000 Cash in checking account Cash in money market account Treasury bill, purchased November 1, 2019 maturing January 31, 2020 Time deposit purchased December 1, 2019 maturing March 31, 2020 3,500,000 4,000,000 What amount s...

Ph of salt solutions worksheet answers
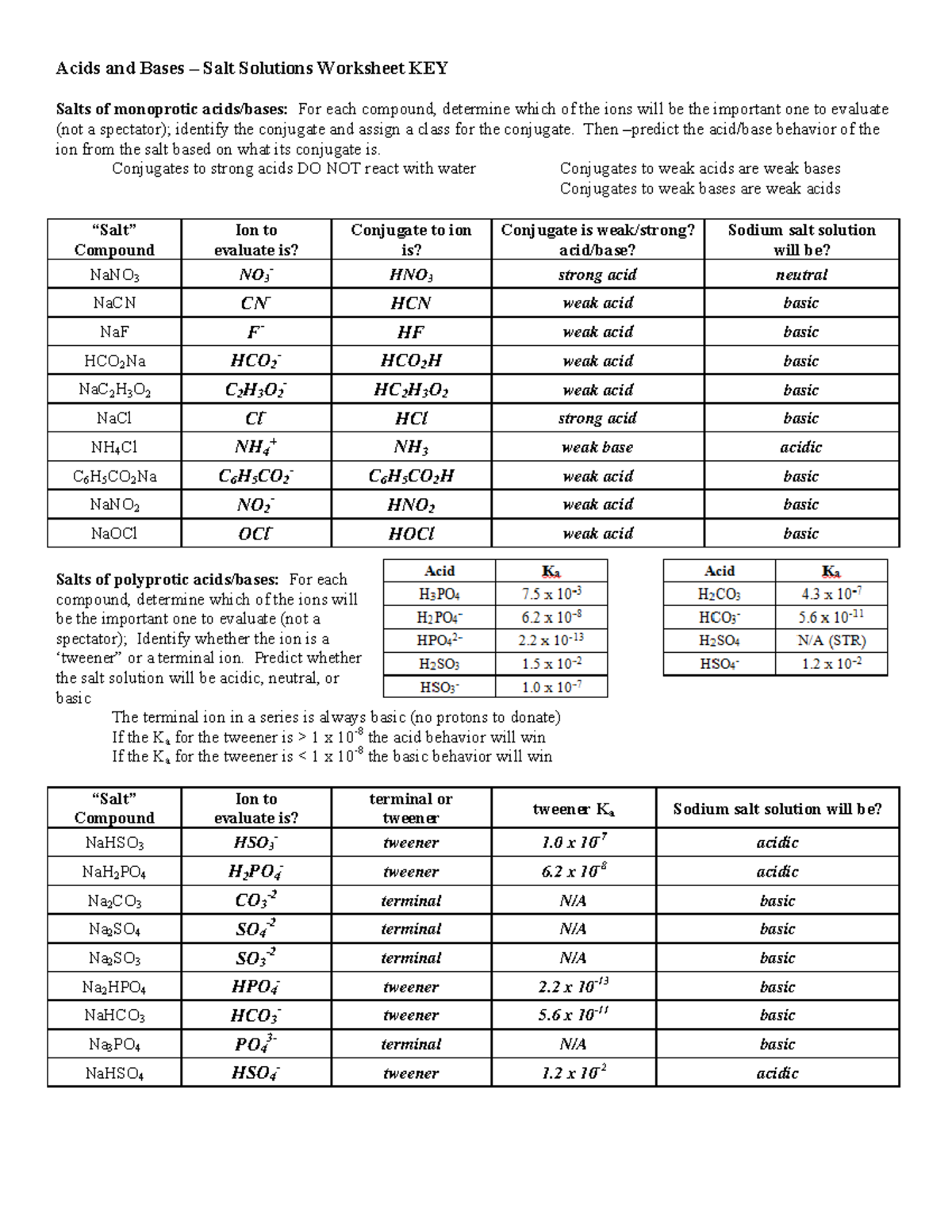
2. What is the pH of a solution containing 0.2 M acetic acid (pKa = 4.7) and 0.1 M sodium acetate? 3. For a weak acid with a pKa of 6.0, show how you would calculate the ratio of acid to salt at pH 5. Ans: 4. Suppose you have just added 100 mL of a solution containing 0.5 mol of acetic acid per liter... Chemistry questions and answers. D. pH of Salt Solutions Salt Solution 0.10 M NaCl 0.10 M Na2CO3 0.10 M Na PO 0.10 M NH,CI 0.10 M Solution:- only week acid and week base undergo a change in water and affect the pH. week acid or base has lower pKa or pKb respectively but strong... Chapter 15 Worksheet 4 (wsI5.4) Acid-Base Properties of Salt Solution, Polyprotic Acids. 4. Calculate the pH of a 0.25 M solution ot1§nonium chlori~lCthis is nothing new). _ Note: For solutions of most weak polyprotic acids, you can get a very good estimate of the pH by using Kat...
Ph of salt solutions worksheet answers. The pH of a salt solution is determined by the relative strength of its conjugated acid-base pair. Salts can be acidic, neutral, or basic. Salts that form from a weak acid and a strong base are basic salts, like sodium bicarbonate (NaHCO3). 2. pH scale, measuring pH, what is an acid?, what is a base?, neutralisation - salt formation, ionic theory of acids & alkalis. This page introduces and explains the pH scale measuring the relative acidity and alkalinity of aqueous solutions, that is solutions of substances dissolved in water. Perform calculations to determine pH of salt solutions if or are known. Keeping things safe and healthy. Sample Problem: Salt Hydrolysis. If we dissolve NaF in water, we get the following equilibrium: The pH of the resulting solution can be determined if the of the fluoride ion is known. As per the title. Essentially, adding salt to raise the dialectic constant to increase the solvating ability of the solution, increasing the propensity of the acid to release a proton.
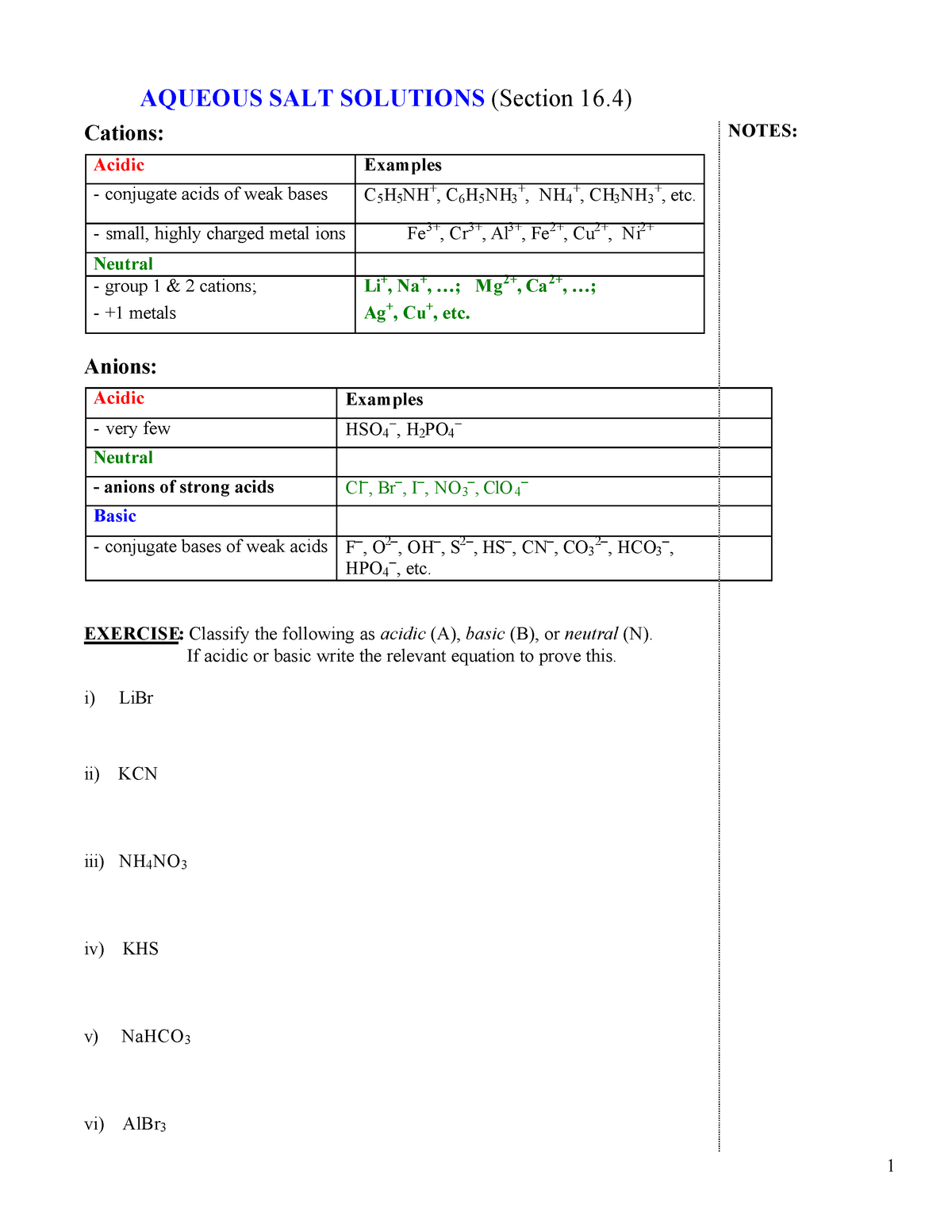
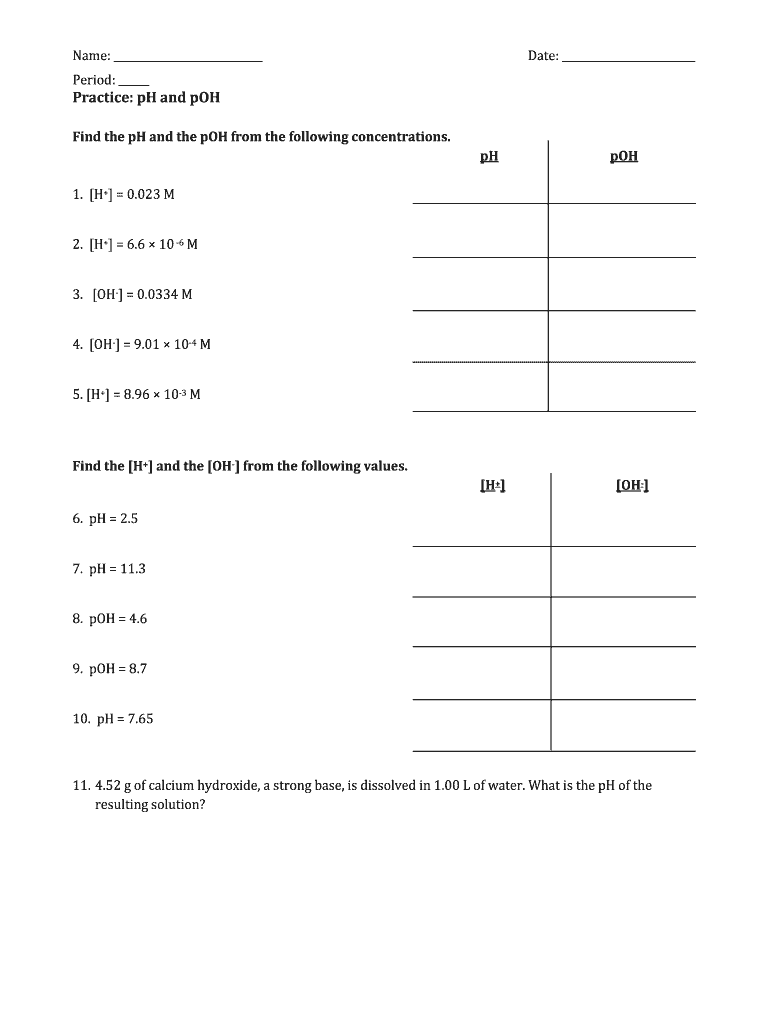
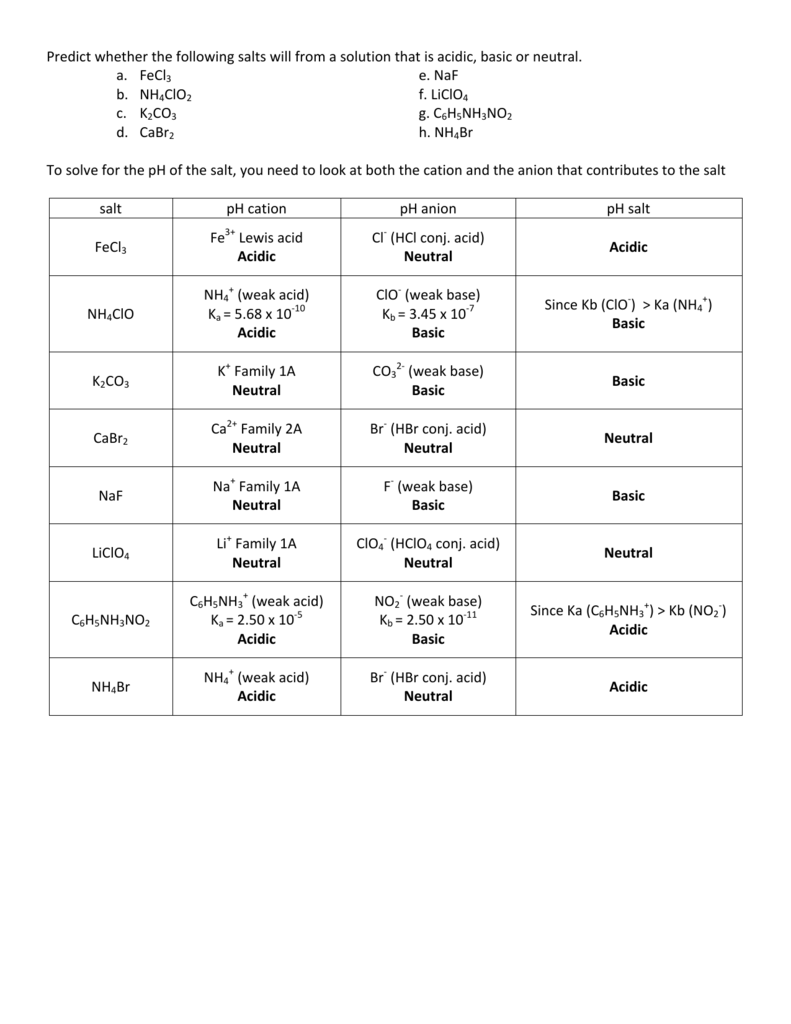
pH of all salts solutions is determined by the hydrolysis and - in the case of acidic or basic salts - by the dissociation. In the case of salt solution Ca=Cb, but we have a good reason to treat these values separately. Once the general equation will be derived we can always simplify it using only one... pH of Two Mixed Solutions. How to Balance Redox Equations in Acidic Solution. Predict whether a salt solution will be acidic, basic, or neutral. Calculate the concentrations of the various Check Your Learning. What is the pH of a 0.083-M solution of NaCN? Answer: 11.11. Calculate the pH of a 0.10-M solution of aluminum chloride, which dissolves completely to give the... Salt solutions may be acidic basic or neutral depending on the original acid and base that formed the salt. Explanation examples or print the worksheet to The ph of a solution is a measure of the molar concentration of hydrogen ions in the solution and as such is a measure of the acidity or. If it has a...
Hello! My professor said that we should prepare our answers or solutions in a worksheet. How should I do it (cash and cash equivalents) in a worksheet? Thank you. Problem 1-10 (AICPA Adapted) Tranvia Company revealed the following information on December 31, 2019: 350,000 750,000 Cash in checking account Cash in money market account Treasury bill, purchased November 1, 2019 maturing January 31, 2020 Time deposit purchased December 1, 2019 maturing March 31, 2020 3,500,000 4,000,000 What amount s... Worksheets are Work 20 polyprotic acids and salt solutions Some of the worksheets for this concept are Work 20 polyprotic acids and salt solutions, Acids bases practice work, Chapter 15 work 4 acid base properties of, Ph of salt solutions, Work to teach balancing equations redox reactions in... 1. You Have Two Solutions, A and B. the pH of Solution A is 6 and pH of Solution B is 8. Which Solution Has More Hydrogen Ion Concentration? The answers to different questions in this section are explained with graphs and practical diagrams concerning a simple comprehension of students. Using ph to calculate k a or pk a p5 conceptual questions. 14.4 hydrolysis of salt solutions; Remind students that the decomposition reaction of hydrogen peroxide and the reaction with copper ii sulfate and aluminum both caused the temperature of the solution to increase. Acids, bases, and conjugates...
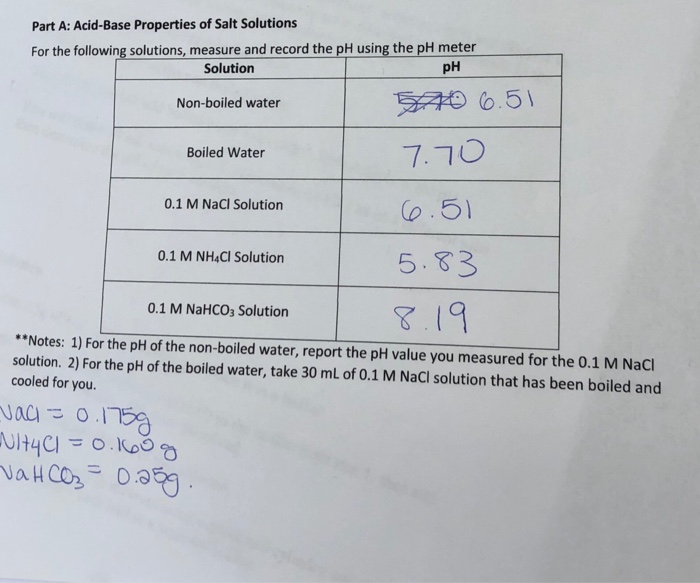
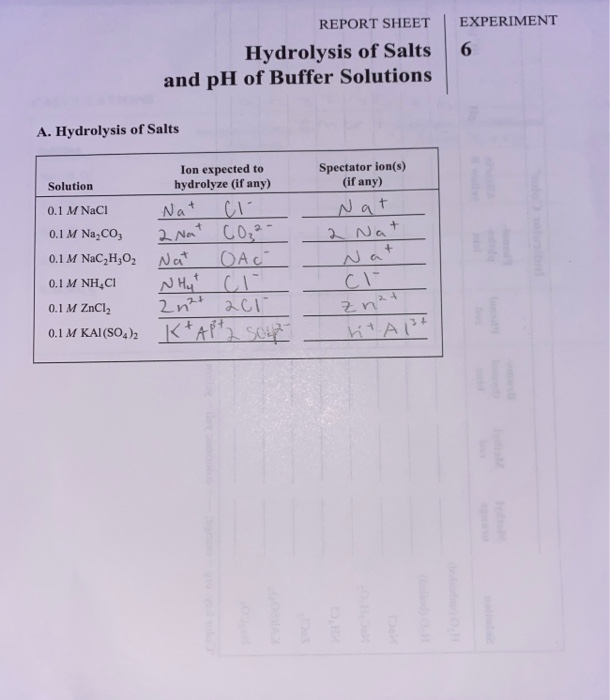
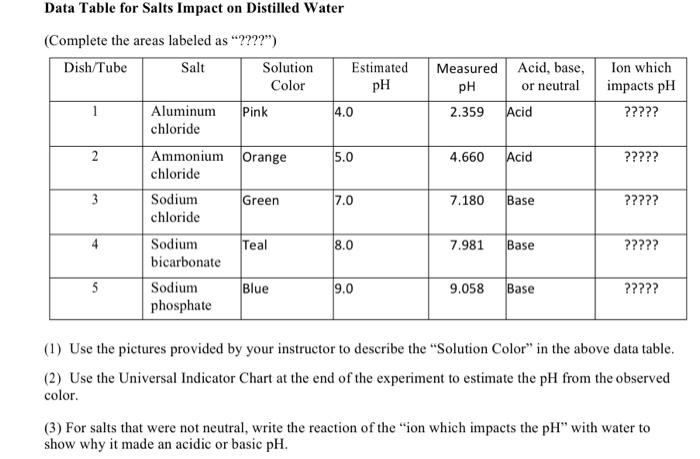
solutions by washing the end of the meter each time it is used. Part 1-‐A. pH of Salt Solutions. Use the recorded pH values of these solutions to write chemical equations for the reaction of the salts with water. For acidic solutions write a chemical equation that shows the production of H3O+, and if...
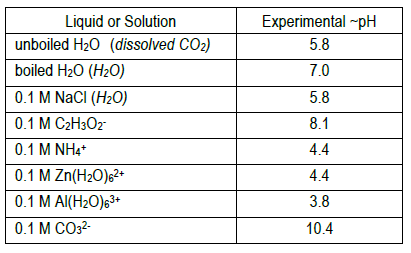
In a dilute salt solution, a soluble salt dissociates completely into its ions. Thus, a water solution labeled "NaBr" actually con-tains Na+ ions and Results from this demonstration show that aluminum chloride and ammonium chloride form acidic solutions in water (pH < 7); sodium chloride forms a...
These worksheets work on understanding the properties of acids and bases in a chemistry based environment. Science Worksheets By Topic. When this occurs salt and water are formed. By the time we are ready for school, we have all heard the about acids and how bad they are.
Water, pH and buffers. When we think of liquid water, we picture lots of H 2 O molecules moving around. Most of the water is like this, but some of the H 2 O molecules disassociate, or split apart ...
Jan 13, 2022 · pH and pH change in Aqueous Solutions: To determine the pH of some fruit juices. Click Here: Unit 6: Titrimetric Analysis: Determination of the concentration (strength) of a given sodium hydroxide solution by titrating it against a standard solution of oxalic acid. Click Here: Unit 7: Systematic Qualitative Analysis: To detect one cation and ...
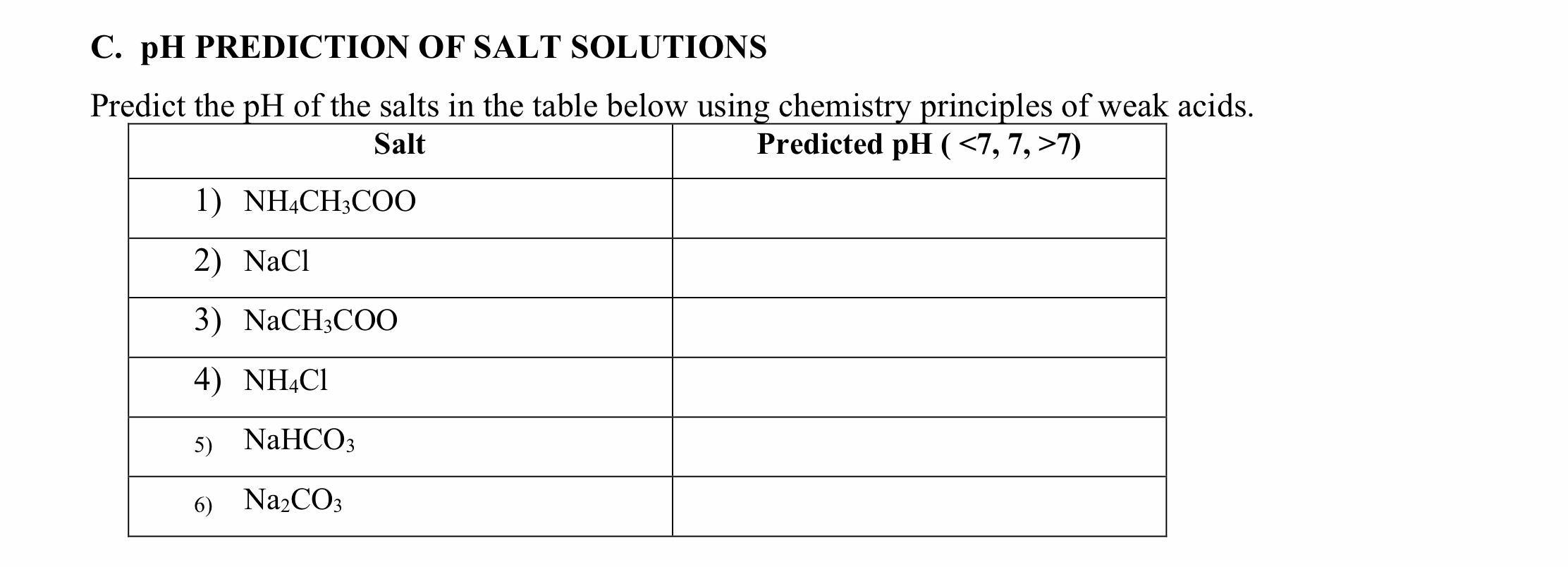
The pH of salt solutions can be measured by universal indicator, also can be determained by calculation. How to calculate the pH of salt solution? In the next activity, you will determain the relation among. Questions No Questions Answer 1 Based on the the strength of parent base and...
Find the concentration of the salt solution, whether the salt is an acidic, basic, or neutral salt, the equation for the interaction of the ion with the water, the equilibrium expression for this interaction and the Ka or Kb value.
So, something is very wrong and yeah chances are it could be me who is totally mistaken. But even if I am, the resources he gave me to learn this is really wrong too, and none of my classmates can get me out of this mess, they don't know how to explain and can't seem to understand my point. So I hope someone here can, before my professor replies to my e-mail (he's talking too long too, to make things worse) ​ **Find the pH of these two solutions a) 0.1 M HCl, b) 10\^-5 M H** I've s...
The pH of a Solution of a Salt of a Weak Base and a Strong Acid Aniline is an amine that is used to manufacture dyes. Answer The pH of the solutions may be calculated using familiar equilibrium techniques, or it may be qualitatively determined to be acidic, basic, or neutral depending on the...
The pH's of eight 0.1M aqueous salt solutions are given below. Write the hydrolysis equation(s) for each. (Several will have more than one equation.) Look at the composition of the salt Salts made from strong acids and strong bases give neutral solutions in water Salts made from strong acids and...
pH of an aqueous solution of a salt of a strong monoprotic acid and weak base is <7 (at 25°C). worksheet wizards to make printable worksheets or tests. self-marking online quizzes for your students. interactive learning activities for students who need a scaffolded approach to problem solving.
Salt Solution Procedure A. Add ~0.5 g of eight different salts to 25 ml water and test with pH paper 1) Use calculate theoretical pH of your solutions III. Buffers A. A Buffer is a solution containing a weak acid and the (For complaints, use another form ). Your e-mail. Input it if you want to receive answer.
Salts That Form Acidic Solutions. When the ammonium ion dissolves in water, the following equilibrium exists Step 3: Think about your results. A salt produced from a strong acid and a weak base yields a solution that is acidic. Video: Finding the pH of a Salt Solution Sample Problem.
Transcribed Image Text. Part C. pH of Salt Solutions 1. Place about 20 drops of the salt solution in a well plate. See Answer. *Response times may vary by subject and question complexity. Median response time is 34 minutes for paid subscribers and may be longer for promotional offers.
The use of dilute salt solutions is a popular method for masking seasonal variation in soil pH. An increase in the soil to water ratio or the presence of salts generally results in a decrease in the soil pH. The pH readings are usually less with dilute salt solutions than with distilled water but may be...
Oct 16, 2017 · B.A., William Paterson College of New Jersey, 1972. M.S., Indiana State University, 1974. M.S. Purdue University, 1979. Ph.D. Purdue University, 1982 (J. Dudley Herron).
Acidic and Basic Salt Solutions. Background Information. Relationship between Ka and Kb of Conjugate Acid-Base Pairs. The equilibrium expression for this reaction can be used to estimate the pH of the salt solution. Since acetate functions as a weak base, the equilibrium constant is given the...
I often use solution books I got somewhere online to guide me while doing my worksheets. For example, if I am facing a tough question, I would check the solution of the question, and try to study it so that I can learn how to answer the question. Another thing I use solution books for is to check if my answers are correct, because I absolutely despise having to do corrections. Is this cheating?
c) pH. Salt of strong acid and weak base. a) Hydrolysis Constant. If pKa < pKb , pH of the solution will be less than 7 and the solution will be acidic. 10Q so much I got the answer of my question.
When is a salt solution basic or acidic? Salts of Polyprotic Acids. Questions. Answers. Salts of weak bases and strong acids do hydrolyze, which gives it a pH less than 7. This is due to the fact that the anion will become a spectator ion and fail to attract the H+, while the cation from the weak base...
Jan 18, 2022 · Parts per million, abbreviated as PPM, is a common term encountered in chemistry. Explore how PPM is measured, how to calculate PPM from a sample, and examples of ratios to practice calculating ...
May 20, 2018 · The solute-solvent interactions require the temperature to decrease further in order to solidify the solution. A common example is found when salt is used on icy roadways. Salt is put on roads so that the water on the roads will not freeze at the normal \(0^\text{o} \text{C}\) but at a lower temperature, as low as \(-9^\text{o} \text{C}\).
If the protein is subject to changes in temperature, pH, or exposure to chemicals, the protein structure may unfold, losing its shape without breaking down the primary sequence in what is known as denaturation (Figure 11.16). Denaturation is different from hydrolysis, in that the primary strcture of the protein is not affected.
A solution will have a pH different from that of the water if either the anion or cation of the salt reacts with water (that's called hydrolysis.) When NaCl dissolves in water it form Na+ ions and Cl- ions, neither of which hydrolyze and change the pH of the resulting solution. The cations (positively charged ions)...
Distillation uses boiling to separate mixtures of liquid solutions. It takes into account that different substances in the mixture will have different boiling points. For example, if you heat salt water the water in the solution will boil before the salt. The water will then evaporate leaving the salt behind.
Chapter 15 Worksheet 4 (wsI5.4) Acid-Base Properties of Salt Solution, Polyprotic Acids. 4. Calculate the pH of a 0.25 M solution ot1§nonium chlori~lCthis is nothing new). _ Note: For solutions of most weak polyprotic acids, you can get a very good estimate of the pH by using Kat...
Chemistry questions and answers. D. pH of Salt Solutions Salt Solution 0.10 M NaCl 0.10 M Na2CO3 0.10 M Na PO 0.10 M NH,CI 0.10 M Solution:- only week acid and week base undergo a change in water and affect the pH. week acid or base has lower pKa or pKb respectively but strong...
2. What is the pH of a solution containing 0.2 M acetic acid (pKa = 4.7) and 0.1 M sodium acetate? 3. For a weak acid with a pKa of 6.0, show how you would calculate the ratio of acid to salt at pH 5. Ans: 4. Suppose you have just added 100 mL of a solution containing 0.5 mol of acetic acid per liter...
























0 Response to "42 ph of salt solutions worksheet answers"
Post a Comment