42 limiting reactant worksheet answers
3. Identify the limiting reactant and determine the mass of CO 2 that can be produced from the reaction of 25.0 g of C 3 H 8 with 75.0 g of O 2 according to the following equation: C 3 H 8 + 5 O 2 3 CO 2 + 4 H 2 O ANS: 61.9 g 4. How many grams of SO 2 are produced when 152 g of CS 2 react with 48.0 g of O 2 according to the following equation: CS 2 Limiting reactant worksheet answers. A 80 0 grams of iodine v oxide i 2 o 5 reacts with 28 0 grams of carbon monoxide co. The reactant that produces a lesser amount of product is the limiting reagent. Show full working in. Stoichiometry Limiting Reagent Worksheet Answers Also Best Limiting Reagent Worksheet Beautiful Chemistry Stoichiometry.
Title: HW - limiting reactant practice answers

Limiting reactant worksheet answers
Limiting and excess reactants worksheet answers pogil It is 1:3. Nitrogen is the limiting reagent. Stoichiometry: Limiting Reagent Problems #1 - 10 Limiting Reagent Worksheet -KEY 1) Write the balanced equation for the reaction given above: CuCl 2 + 2 NaNO 3 Cu(NO 3) 2 + 2 NaCl 2) If 15 grams of copper (II) chloride react with 20 grams of sodium nitrate, how much sodium chloride 10. 51.78 grams of magnesium hydroxide are sued to neutralize 52.00 grams of hydrochloric acid. What is the limiting reactant? What is the mass of each product formed? What is the mass of excess reactant? Honors Chemistry Worksheet on Limiting Reactants. Show all work in solving the following problems. Express any equations used. ANSWERS to Practice Problems on "Limiting Reactant" and % yield handout (from Chapter 4 in "Chemistry, the Molecular Science", Moore, Stanitski, and Jurs (2002, Harcourt). 57. CO(g) + 2 H 2 (g) CH 3 OH(l) (a) Starting with 12.0 g H 2 and 74.5 g CO, which is limiting? ANS: CO is the L.R.. Convert to moles first: 2 2 2 12.0 g H = 5.952 mol H
Limiting reactant worksheet answers. Answers to worksheet 14 limiting reagents a limiting reagent is the reactant that is completely used up in a reaction. Limiting reactant worksheet answers along with fresh limiting reactant worksheet fresh percent yield and limiting. B if in the above situation only 0 160 moles of iodine i 2 was produced. Worksheet. 1. Say you take a reactant A and calculate the amount of moles of another reactant B required to use up all of A. How do you know which of two reactants is the limiting one? You compare ... Worksheet on Limiting Reactants. Use the following equation to answer questions 1-4. N2 + H2 ( NH3 . 1. How many moles of NH3 can be produced from the reaction of 28 g of N2 ? 2. How many moles of NH3 can be produced from the reaction of 25 g of H2? 3. If 28 g of N2 and 25 g of H2 are reacted together, which one would be the limiting reactant? November 22, 2021 on Limiting Reactant Practice Problems With Answers Pdf. Theoretical Actual And Percent Yield Problems Chemistry Tutorial Chemistry Chemical Reactions Tutorial. 2 00g Prelab Chemistry Writing Presentation. A Simple Guide On How To Calculate Theoretical Yield Detox Fast Wine Folly Wine Map. Physical Chemical Change Work Sheet ...
Name _____ Limiting Reagent Worksheet 1) When copper (II) chloride reacts with sodium nitrate, copper (II) nitrate and sodium chloride are formed. a) Write the balanced equation for the reaction given above: b) If 15 grams of copper (II) chloride react with 20 grams of sodium nitrate, how much sodium chloride Limiting reagents and percentage yield worksheet 1. Limiting reactant and percent yield worksheet answers. Ii what percentage yield of iodine was produced. 80 g i2o5 1 mol i2o5 1 mol i2 xs 1 333 8 g i2o5 1 mol i2o5 28 g co 1 mol co. Co g 2 h 2 g ch 3 oh l answer. This reagent is the one that determines the amount of product formed. Limiting Reactant and Percent Yield Practice Name_____ 1) Consider the following reaction: NH 4 NO 3 + Na 3 PO 4 (NH 4) 3 PO 4 + NaNO 3 Which reactant is limiting, assuming we started with 30.0 grams of ammonium nitrate and 50.0 grams of sodium phosphate. What is the mass of each product that can be formed? What is the limiting reactant. Also show how much of the other reactant the reactant in excess will be left over. Indicate which reactant limits the quantity of water produced this is the limiting reactant. Worksheet 14 3 answers to worksheet 14 limiting reagents a limiting reagent is the reactant that is completely used up in a reaction.
Stoichiometry 3.5 Limiting Reactant Worksheet A 24.5g sample of sodium chloride reacts with 41.3g of fluorine gas according to the following chemical equation: 2 + -¥ 2NaF(s) + Cl Worksheet 14 3 answers to worksheet 14 limiting reagents a limiting reagent is the reactant that is completely used up in a reaction. Consider the reaction i2o5 g 5 co g 5 co2 g i2 g a 80 0 grams of iodine v oxide i2o5 reacts with 28 0 grams of carbon monoxide co. The volume and molarity given have to be employed to get the number of moles of hcl. What is the limiting reactant? ... Limiting Reagent Worksheet -KEY. All of the questions on this worksheet involve the following reaction: When copper (II) chloride reacts with sodium nitrate, copper (II) nitrate and sodium chloride are formed. ... Since the smallest of the two answers is 8.51 grams, this is the quantity of sodium nitrate that ... Practice Problems: Limiting Reagents (Answer Key) Take the reaction: NH 3 + O 2 NO + H 2 O. In an experiment, 3.25 g of NH 3 are allowed to react with 3.50 g of O 2.. a. Which reactant is the limiting reagent?
The substance that has the smallest answer is the limiting reagent. Limiting Reactant and Percent Yield Worksheet Answer Key or 24 Fresh Stoichiometry Limiting Reagent Worksheet. Observing a limiting reactant lab answers are a great way to get information regarding operating certain products.
In one experiment 0.866 mol of NO is mixed with 0.503 mol of O2. a)Determine the limiting reagent. b) Calculate the number of moles of NO2 produced. This is a limiting reagent problem. Let's calculate the moles of NO2 produced assuming complete reaction for each reactant. 2NO(g) O2(g) ( 2NO2(g)
• Limiting-reactant principle - The maximum amount of product possible from a reaction is determined by the amount of reactant present in the least amount, based on its reaction ... To answer this question, calculate the grams of NO 2 needed to react fully with 79.6 grams of NO and 59.5 grams of O 2, by using the balanced equation.
15.0g of each reactant is used. What is the limiting reactant? 2Al (s) + 6HCl (aq) → 2AlCl 3(aq) + 3H 2(g) Start with either reactant and solve for the mass of the other reactant; € 15.0gAl 1 x 1molAl 27gAl x 6molHCl 2molAl x 36gHCl 1molHCl =60.0gHCl, we do not have 60.0g of HCl available
Limiting reactant and percent yield worksheet answers. Determine the mass of iodine i2 which could be produced. Consider the reaction i 2 o 5 g 5 co g 5 co 2 g i 2 g a 80 0 grams of iodine v oxide i 2 o 5 reacts with 28 0 grams of carbon monoxide co. 8 8 to react. The volume and molarity given have to be employed to get the number of moles of ...
Limiting Reagent Worksheet W 324 Everett Community College Student Support Services Program 1) Write the balanced equation for the reaction that occurs when iron (II) chloride is mixed with sodium phosphate forming iron (II) phosphate and sodium chloride. 2) If 23 grams of iron (II) chloride reacts with 41 grams of sodium
Limiting reactant worksheet answers. A limiting reactant is a reagent that s completely consumed throughout a chemical. Nitrogen is the limiting reagent. What is the limiting reactant. What is the limiting reactant. Hw limiting reactant practice answers. Given the following reaction.
a) Which chemical is the limiting reactant? Zn b) How many grams of ZnS will be formed? 0.3803 mol = 37.1 g c) How many grams of the excess reactant will remain after the reaction is over? 17.7 g 3. Which element is in excess when 3.00 grams of Mg is ignited in 2.20 grams of pure oxygen? O 2 What mass is in excess? 0.226 g O
Limiting reactant and percent yield worksheet answers. Co g 2 h 2 g ch 3 oh l answer. Consider the reaction i 2 o 5 g 5 co g 5 co 2 g i 2 g. Limiting reagent calculations are performed in the same manner as the stoichiometric equations on worksheet 11. Determine the mass of iodine i2 which could be produced.
Limiting Reagent Worksheet #1 1. Given the following reaction: (Balance the equation first!) C 3H 8 + O 2-----> CO 2 + H 2O a) If you start with 14.8 g of C 3H 8 and 3.44 g of O 2, determine the limiting reagent b) determine the number of moles of carbon dioxide produced c) determine the number of grams of H 2O produced
Worksheet 14 3 Answers to Worksheet #14 Limiting Reagents A Limiting Reagent is the reactant that is completely used up in a reaction. This reagent is the one that determines the amount of product formed. Limiting reagent calculations are performed in the same manner as the stoichiometric equations on Worksheet #11. However, with a limiting
ANSWERS to Practice Problems on "Limiting Reactant" and % yield handout (from Chapter 4 in "Chemistry, the Molecular Science", Moore, Stanitski, and Jurs (2002, Harcourt). 57. CO(g) + 2 H 2 (g) CH 3 OH(l) (a) Starting with 12.0 g H 2 and 74.5 g CO, which is limiting? ANS: CO is the L.R.. Convert to moles first: 2 2 2 12.0 g H = 5.952 mol H
10. 51.78 grams of magnesium hydroxide are sued to neutralize 52.00 grams of hydrochloric acid. What is the limiting reactant? What is the mass of each product formed? What is the mass of excess reactant? Honors Chemistry Worksheet on Limiting Reactants. Show all work in solving the following problems. Express any equations used.
Limiting and excess reactants worksheet answers pogil It is 1:3. Nitrogen is the limiting reagent. Stoichiometry: Limiting Reagent Problems #1 - 10 Limiting Reagent Worksheet -KEY 1) Write the balanced equation for the reaction given above: CuCl 2 + 2 NaNO 3 Cu(NO 3) 2 + 2 NaCl 2) If 15 grams of copper (II) chloride react with 20 grams of sodium nitrate, how much sodium chloride

PRISON of the MIND - most people are living in an invisible prison made of their own thoughts and beliefs. 18 metre tall Big Buddha statue at Wat Phra Yai temple is getting a fresh golden coating.

I bought strawberries on sale today at our local market, I plan to use them to make strawberry shortcake. There was an employee just outside of the market counting people as they went inside using a clicker, they are limiting it to 100 people at a time because of social distancing due to the Covid-19 pandemic. When they reach the limit, people must wait outside until some leave before more are allowed in. They also closed off the second entrance, so that all foot traffic must go in and out of the same entrance. The market had no toilet paper, no flour, and very little pasta. I needed flour...





























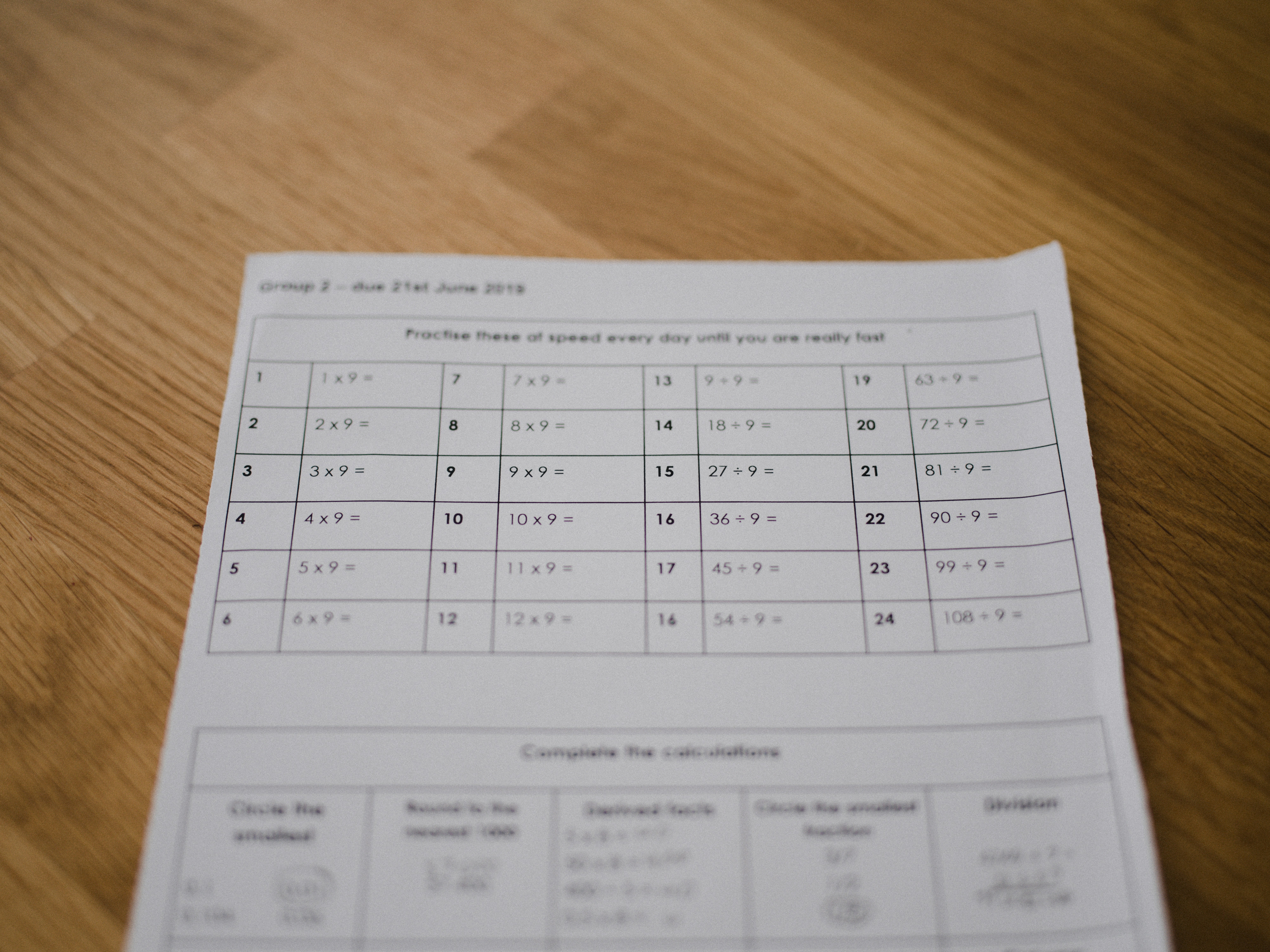




0 Response to "42 limiting reactant worksheet answers"
Post a Comment