40 chemistry ef and mf worksheet answers
CHEMISTRY E.F. AND M. F. Worksheet ... To determine the molecular formula you set the EF mass taken X times and set it equal to the Molecular Weight of the compound. Like this: ... You can see from this example that the MF is a multiple of the EF and represents the actual number of each type of atom that makes up the compound. Empirical and Molecular Formula Worksheet Answer the following questions: 1. The percentage composition of acetic acid is found to be 39.9% C, 6.7% H, ...
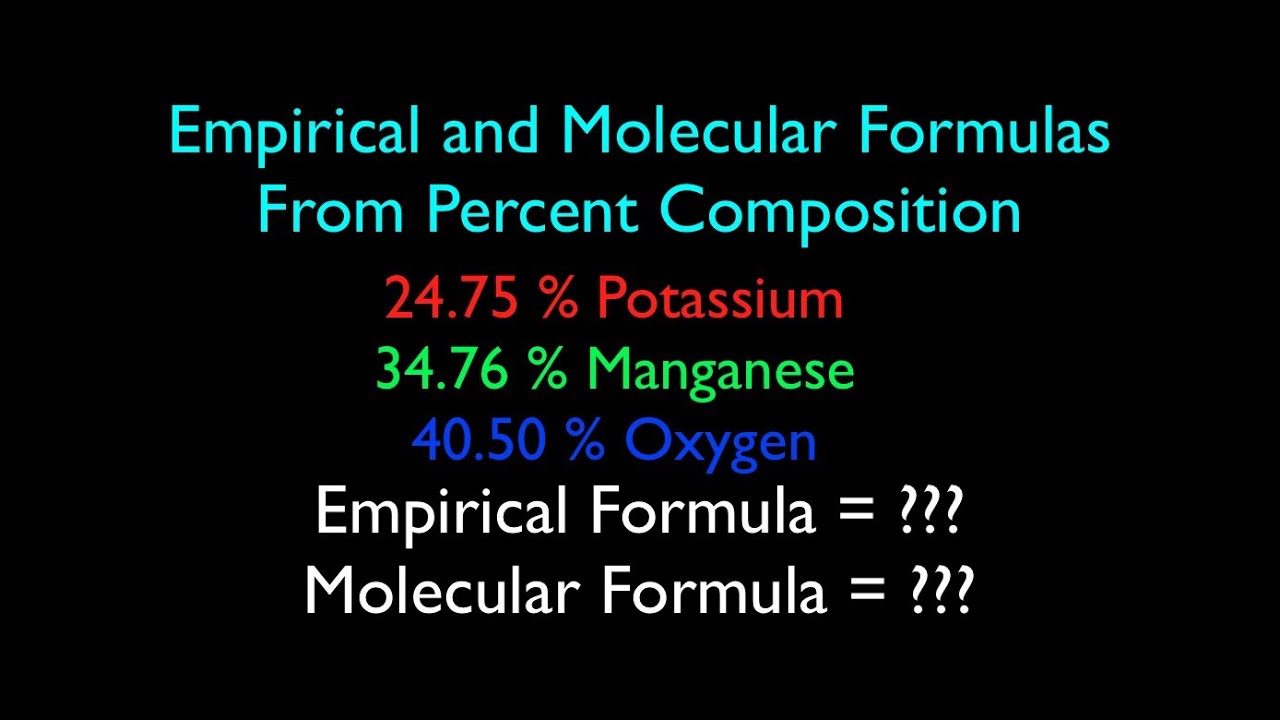
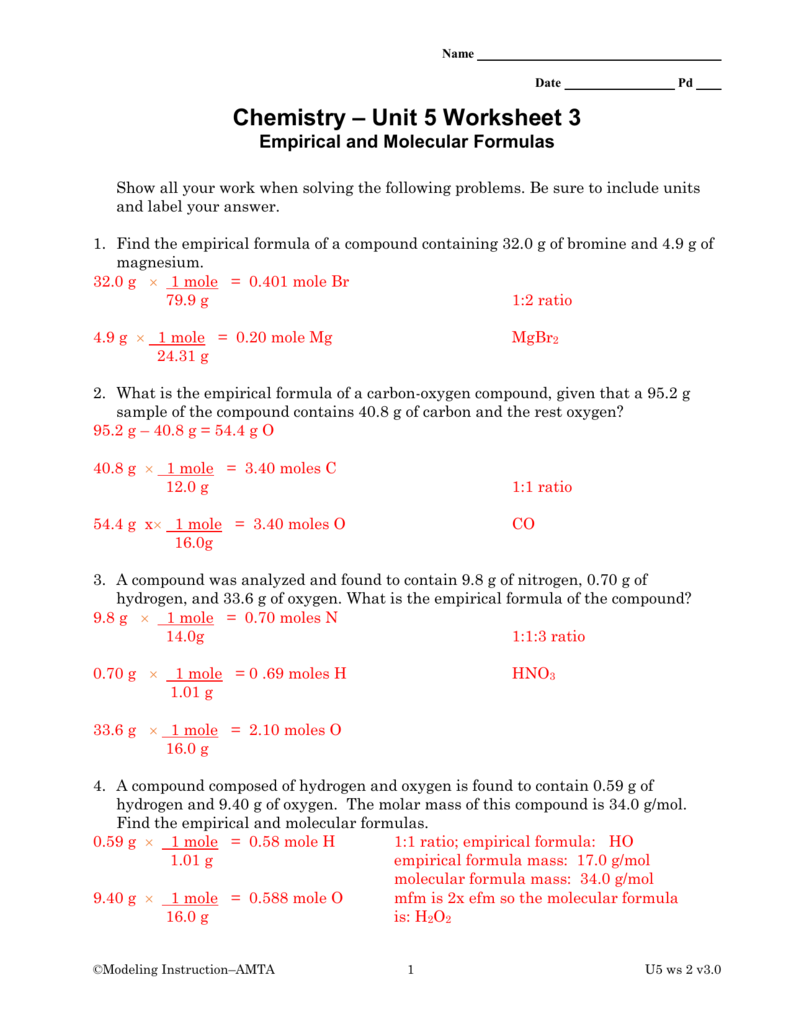
EMPIRICAL AND MOLECULAR FORMULA WORKSHEET 1. An oxide of chromium is found to have the following % composition: 68.4 % Cr and 31.6 % O. Determine this compound's empirical fomula. I $15 CX2Ô3 2. The percent composition of a compound was found to be 63.5 % silver, 8.2 % nitrogen, and 28.3 % oxygen. Determine the compound's empirical formula. 3.

Chemistry ef and mf worksheet answers
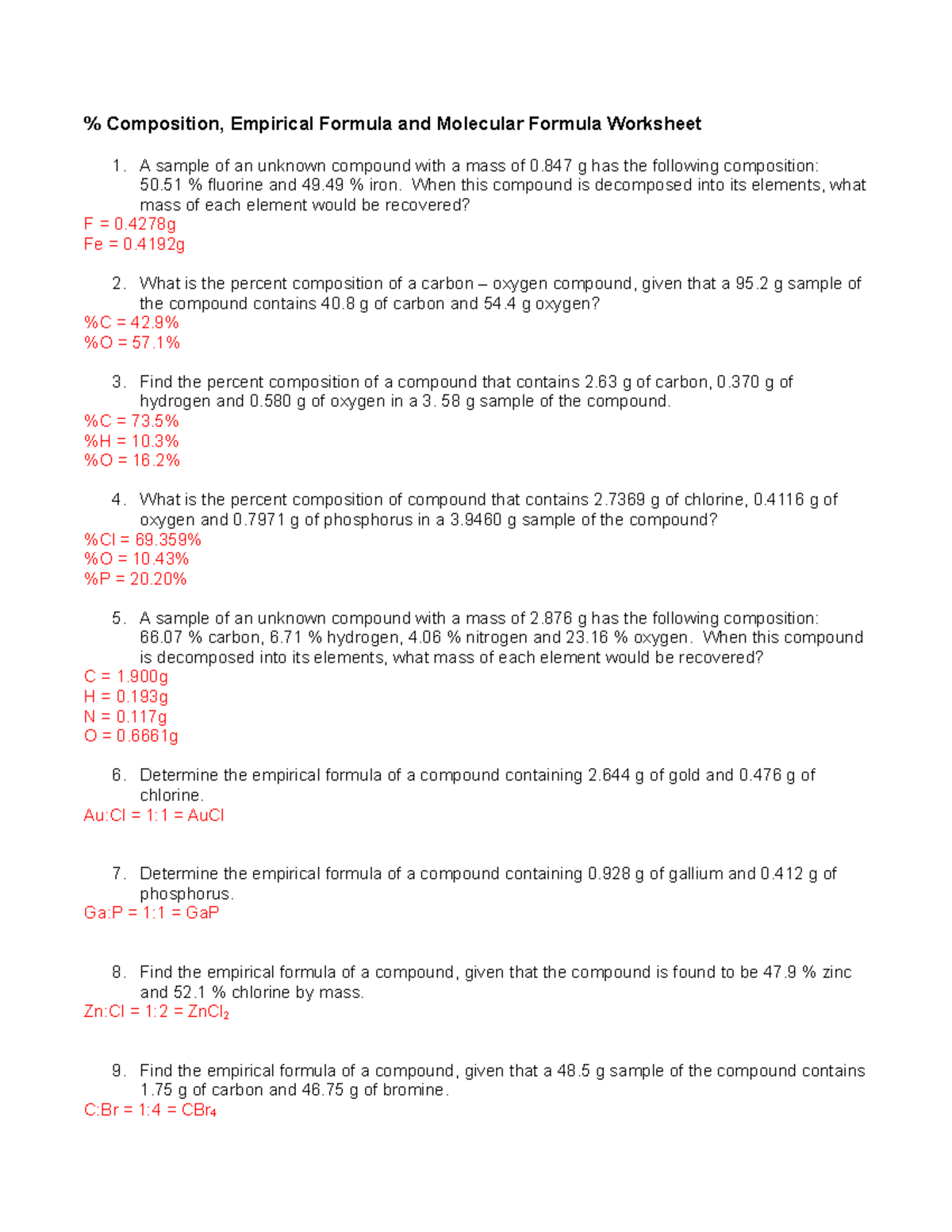
Composition, Empirical Formula and Molecular Formula Worksheet Be sure to show all work! Answers are in brackets beside the question. 1. Honors Chemistry Name _____ HW – Hydrates, Percent Composition, EF/MF Date _____ Complete these problems on a separate sheet of paper. 1. Determine the percent Oxygen in Potassium Perchlorate. KClO4 Molar Mass is 138.547 g KClO4 4 moles of O in KClO4 Molar mass of O is 15.999 Answers are in parentheses at the end of some problems. 38. What is the empirical formula for a compound containing only carbon and hydrogen if it is known to contain 84.2% carbon? (Answer: C4H9) 39. Find the empirical formula of a compound that contains 15.8 g of Al, 28.1 g of S, and 56.1 g of oxygen. (Answer: A12S3012 or 40.
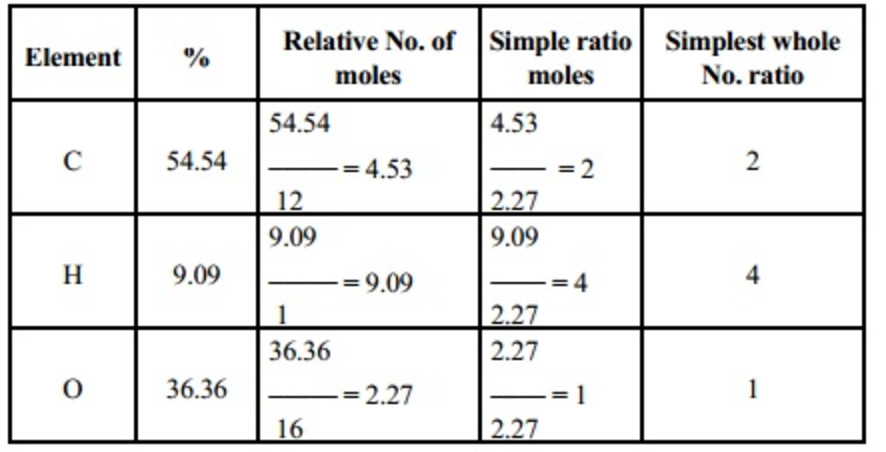
Chemistry ef and mf worksheet answers. View EF & MF Worksheet (3).pdf from CHEM 1131 at Durham College. EMPIRICAL AND MOLECULAR FORMULA WORKSHEET An oxide of chromium is found to have the following % composition: 68.4 % Cr and 31.6 % 0. The molar mass of the compound is 92 g/mol. (EF: NO 2 , MF: N 2 O 4 ) Ninhydrin is a compound that reacts with amino acids and proteins to produce a dark-col- ored complex. It is used by forensic chemists and detectives to see fingerprints that might other- wise be invisible. Honors Chemistry Molecular Formula Worksheet – From EF and MM of MF 1. State the empirical formula for each of the following compounds with molecular formulas: a) C 4 H 8 x = 4 b) C 2 H 6 O 2 c) N 2 O 5 x = 1 d) Ba 3 (PO 4) 2 e) Te 4 I 16 a) CH 2 b) _____ c) N 2 O 5 11. Determine the (MF mass)/ (EF mass) ratio for each substance and enter it into Column B of the table in Model 2. Be sure that your entire group agrees on the values. 12. Look at the Information in Column C and Column D that is given for ethyne and ethene and write a complete
EF = PdH2. Multiple = 2. MF = Pd2H4. Octane, a compound of hydrogen and carbon, has a molar mass of 114.26 g/mol. If one mole of the compound contains 18.17 g of hydrogen, what is its molecular formula? C8H18. Find the molecular formula of a compound that contains 30.45 % nitrogen and 69.55 % oxygen. The molar mass of the compound is 92.02 g ... EMPIRICAL FORMULA & MOLECULAR FORMULA WORKSHEET 1. Find the molecular formula of a compound that has a molar mass of 26 g/mol, and has an empirical formula of CH. EF is CH, mass = 12 + 1 = 13 g/mol MF = n (EF) n = molar mass ÷ empirical mass, = 26 ÷ 13 = 2. So MF = C 2 H 2 2. Answers are in parentheses at the end of some problems. 38. What is the empirical formula for a compound containing only carbon and hydrogen if it is known to contain 84.2% carbon? (Answer: C4H9) 39. Find the empirical formula of a compound that contains 15.8 g of Al, 28.1 g of S, and 56.1 g of oxygen. (Answer: A12S3012 or 40. Honors Chemistry Name _____ HW – Hydrates, Percent Composition, EF/MF Date _____ Complete these problems on a separate sheet of paper. 1. Determine the percent Oxygen in Potassium Perchlorate. KClO4 Molar Mass is 138.547 g KClO4 4 moles of O in KClO4 Molar mass of O is 15.999
Composition, Empirical Formula and Molecular Formula Worksheet Be sure to show all work! Answers are in brackets beside the question. 1.




















/chemical-periodic-table-of-elements-with-color-cells-vector-illustration-840464844-ff61af51081b4fb1959504105e232e01.jpg)
0 Response to "40 chemistry ef and mf worksheet answers"
Post a Comment