39 chemistry counting atoms in compounds worksheet
Counting Atoms Using the Mole . ... Food Chemistry: Quiz & Worksheet for Kids . View Quiz. ... Physical Properties of Organic Compounds . View Quiz. Organic Compound Names . substances are made by combining atoms in various ways to make molecules. When a chemical reaction takes place the atoms are rearranged to make different molecules but no atoms can be made or destroyed. To show this you have to be able to find a method of counting the atoms that take part in a reaction and its products.
This paper (doc) will be needed through chapters 6-8 and needed for your chemistry final. CHEMISTRY: COUNTING ATOMS IN COMPOUNDS WORKSHEET #7.0.1. INSTRUCTIONS: Write the quantity of atoms of each element opposite the formula of the compound for ...16 pages. CHEMISTRY: COUNTING ATOMS IN COMPOUNDS WORKSHEET #7.0.1.
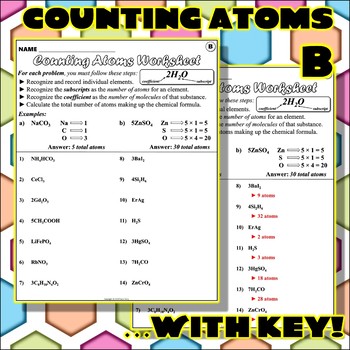
Chemistry counting atoms in compounds worksheet
The mole is the "counting unit" used by chemists to indicate the number of atoms, ions, ... Notice that you must be very careful when you're counting the number of atoms present in ... Calculate the molar mass for each of the following compounds: 1. Fe 2O 3 2. AgNO 3 3. Pb(Cr 2O 7) 2 4. Ca(ClO 4) 2. 1. The molar mass of Fe 2O Try these ones… Count the number of atoms of each element in the following compounds: CuSO 4 Li 2CO 3. Al 2(SO 4) 3 4Na 2CO 3. Elements Present . Number of Atoms Avogadro’s number is defined as the number of elementary particles (molecules, atoms, compounds, etc.) per mole of a substance. It is equal to 6.022×10 23 mol -1 and is expressed as the symbol N A .
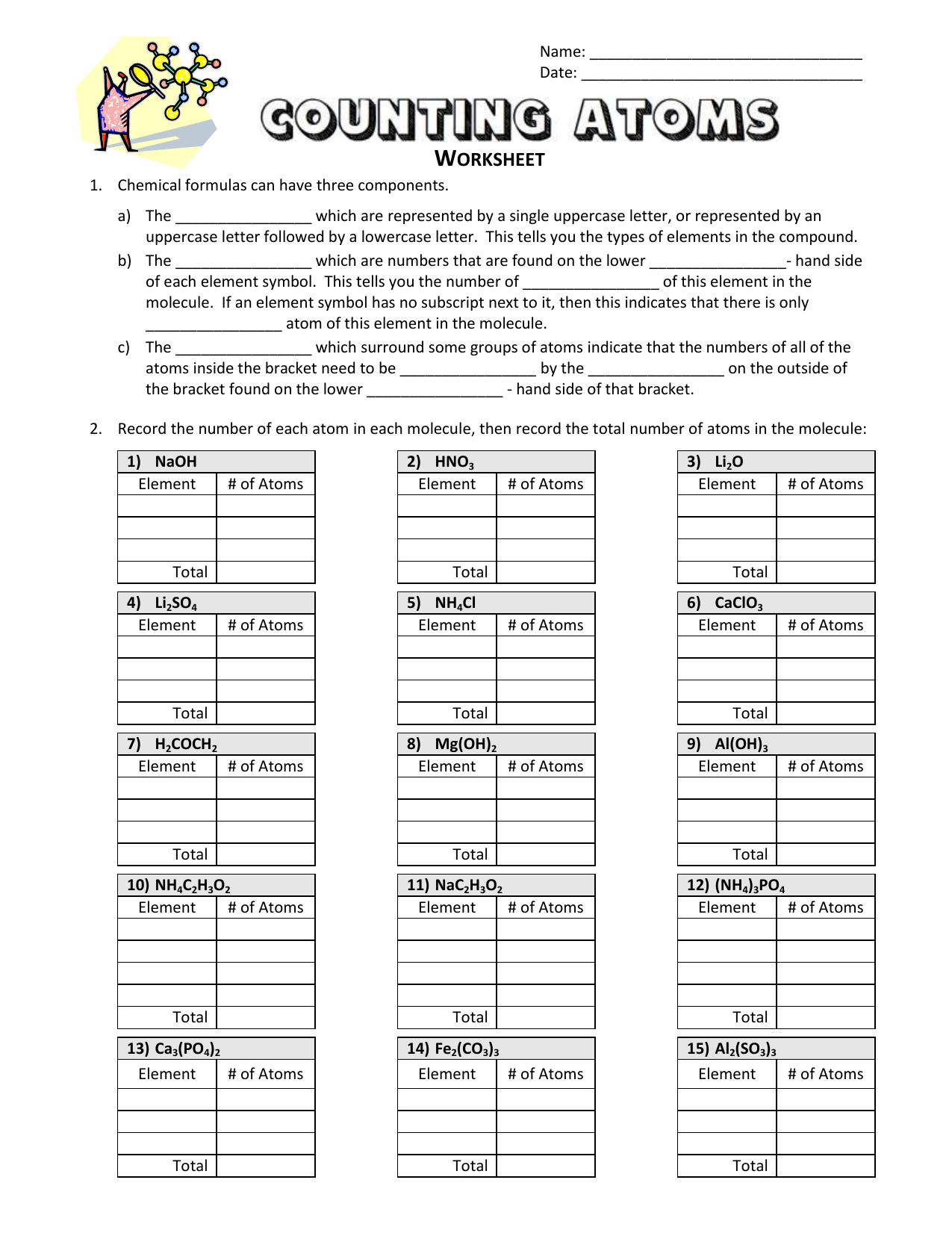
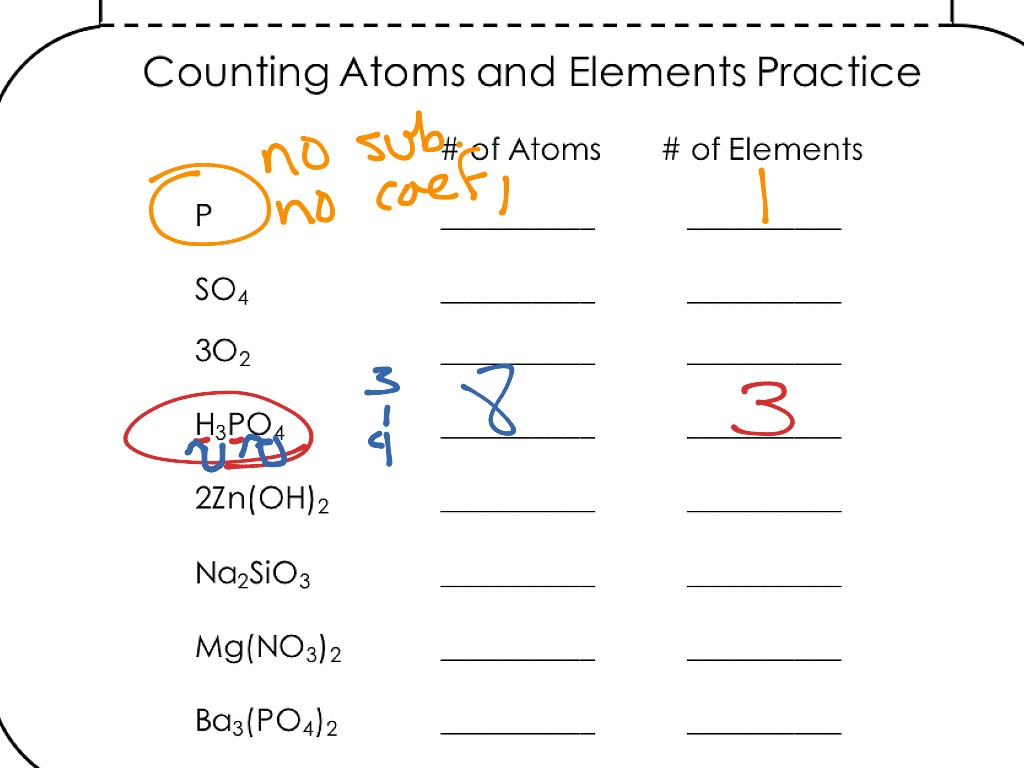
Chemistry counting atoms in compounds worksheet. CHEMISTRY: COUNTING ATOMS IN COMPOUNDS WORKSHEET #7.0.1 INSTRUCTIONS: Write the quantity of atoms of each element opposite the formula of the compound for the quantity of formula units and molecules shown: For example: 5P 2O 3 P = (5 + 2 = ) 10 O = (5 + 3 = ) 15 For example: 4Zn(NO 3) My compound is Count the atoms in one molecule H atoms C atoms O atoms N atoms Na atoms How many atoms are in one molecule of your compound? Now to determine the identity of a mystery compound, we must count the number and types of atoms in a molecule. Count the atoms in one molecule 10 H atoms C atoms O atoms N atoms Na atoms Chemistry Help and Problems In our chemistry help section, you'll find a broad range of topics from very basic chemistry all the way through some more advanced organic chemistry topics. Browse our chemistry topics below, or contact one of our chemistry tutors for private help. Heuristics Before you get into the other topics, learning HOW… Counting Atoms Worksheet List the number of atoms of each element within the compound below. C = 2 O = 4 Compound Atoms in Compound NaCl Na = 1 Cl = 1 BaCl 2 Ba = Cl = LiBr FeS 2 BaSO 4 Ba = O= S = CaSO 4 3CaCO 2 C 6 H 4 Cl 2 C 2 H 4 O 2 Mg(OH) 2 C 7 H 5 (NO 2) 3 2 Ca(H 2 PO 4) 2 2HBr 3H 2 0 2C 2 0 2
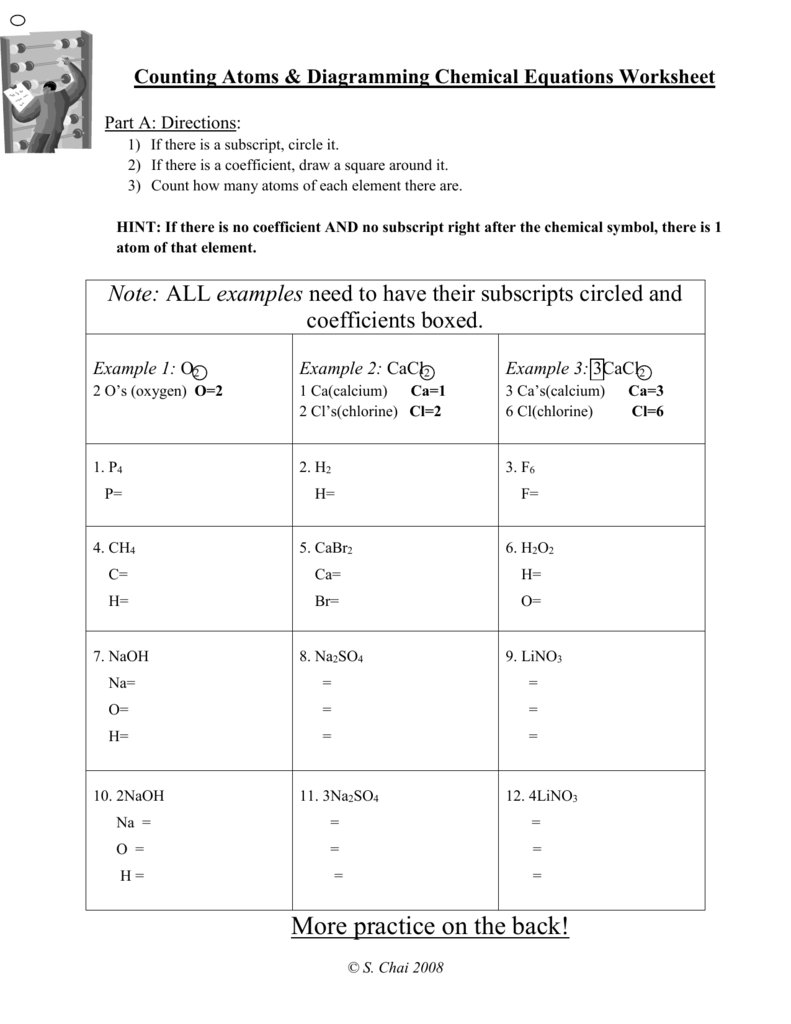
They can be atoms, formula units (of ionic compounds), or molecules. That information still needs to be specified. Because 1 H 2 molecule contains 2 H atoms, 1 mol of H 2 molecules (6.02 × 10 23 molecules) has 2 mol of H atoms. Using formulas to indicate how many atoms of each element we have in a substance, we can relate the number of moles ... CHEMISTRY: COUNTING ATOMS IN COMPOUNDS WORKSHEET #7.0.1 INSTRUCTIONS: Write the quantity of atoms of each element opposite the formula of the compound for the quantity of formula units and molecules shown: For example: 5P2O3 P = (5 H 2 = ) 10 O = (5 H 3 = ) 15 Counting Atoms Worksheet Count the atoms present in the different compounds by using the coefficients and subscripts. K2C03 Type of Atom Total Na2Cr04 Type of Atom So Total NH4C2H302 Type of Atom Total Type of Atoms Total # of Atoms # of Atoms # of Atoms # of Atoms Ty e of Atom Total 3 caC12 Type of Atom Type of Atom Total 2 (NH4)2Cr207 Mar 24, 2021 · 2. Arrange the atoms to show specific connections. Because carbon is less electronegative than oxygen and hydrogen is normally terminal, C must be the central atom. 3. Place a bonding pair of electrons between each pair of adjacent atoms to give a single bond. Placing a bonding pair of electrons between each pair of bonded atoms gives the ...
Oct 25, 2021 · Organic Chemistry Multiple Choice Questions Highlights – 1000 Multiple Choice Questions Answers MCQs in Organic Chemistry with a detailed explanation of every question. You need to learn to name them according to the IUPAC or International Union of Pure and Applied Chemistry which is the currently. Determining Empirical Formulas Worksheet School … Nov 07, 2021 · Because one molecule of n-heptane contains 16 hydrogen atoms, we need 8 H2O molecules, each of which contains 2 hydrogen atoms, on the right side: \[ \ce{C_7H_{16} + O_2 \rightarrow 7CO_2 + 8H_2O} \label{3.1.5}\] The carbon and hydrogen atoms are now balanced, but we have 22 oxygen atoms on the right side and only 2 oxygen atoms on the left. View countingatomsworksheet.pdf from CHEMISTRY 123 at Northwest Cabarrus High. 3rd BLOCK: _ DATE: _ Raghad Sayed NAME: _ CHEMISTRY: COUNTING ATOMS IN COMPOUNDS WORKSHEET #7.0.1 INSTRUCTIONS: Write CHEMISTRY: COUNTING ATOMS IN COMPOUNDS WORKSHEET INSTRUCTIONS: Write the quantity of atoms of each element opposite the formula of the compound for the quantity of formula units and molecules shown: 24 For example: 5P203 For example: (5x3=ì15 4. 10. 13. 15. 16. 18. 4K2C03 3N40i0 8C120 12NaBr 3NaHC03 4Fe203 6Ba(Mn04)2 2KN03 9MgS04 Sr = Ca = Al ...
Ionic compounds are composed of discrete cations and anions combined in ratios to yield electrically neutral bulk matter. The formula mass for an ionic compound is calculated in the same way as the formula mass for covalent compounds: by summing the average atomic masses of all the atoms in the compound’s formula.
Chemistry Counting Atoms In Compounds Worksheet. Utilize the populated lines to lead them in eliminating this fish image. The moment is your own, so do not stress and anxiety concerning it. When children are hurried, they are most likely to wound themselves with scissors. Provide lots of time to reach a safe place.
Avogadro’s number is defined as the number of elementary particles (molecules, atoms, compounds, etc.) per mole of a substance. It is equal to 6.022×10 23 mol -1 and is expressed as the symbol N A .
Try these ones… Count the number of atoms of each element in the following compounds: CuSO 4 Li 2CO 3. Al 2(SO 4) 3 4Na 2CO 3. Elements Present . Number of Atoms
The mole is the "counting unit" used by chemists to indicate the number of atoms, ions, ... Notice that you must be very careful when you're counting the number of atoms present in ... Calculate the molar mass for each of the following compounds: 1. Fe 2O 3 2. AgNO 3 3. Pb(Cr 2O 7) 2 4. Ca(ClO 4) 2. 1. The molar mass of Fe 2O

































0 Response to "39 chemistry counting atoms in compounds worksheet"
Post a Comment