39 atomic mass and atomic number worksheet answers
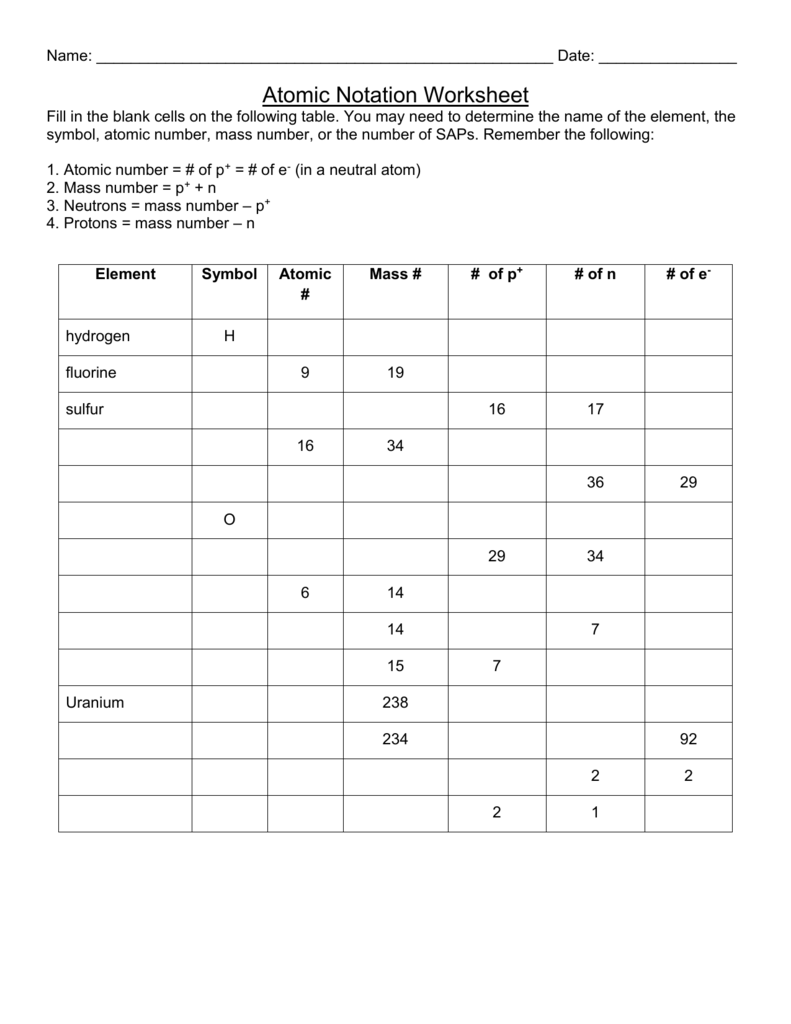
Atomic Mass and Atomic Number Worksheet - Key Name of Element Symbol Atomic Number Atomic Mass Protons Neutrons Electrons copper Cu 29 64 29 35 29 tin Sn 50 119 50 69 50 iodine I 53 127 53 74 53 uranium U 92 238 92 146 92 potassium K 19 39 19 20 19 lithium Li 3 7 3 4 3 oxygen O 8 16 8 8 8 ... No information is available for this page.Learn why
The atomic number or proton number (symbol Z) of a chemical element is the number of protons found in the nucleus of every atom of that element. The atomic number uniquely identifies a chemical element.It is identical to the charge number of the nucleus. In an uncharged atom, the atomic number is also equal to the number of electrons.. The sum of the atomic …
Atomic mass and atomic number worksheet answers
Example Exercise 9.1 Atomic Mass and Avogadro's Number. The atomic mass of each element is listed below the symbol of the element in the periodic table: Cu = 63.55 amu, Hg = 200.59 amu, S = 32.07 amu, and He = 4.00 amu. The mass of Avogadro's number of atoms is the atomic mass expressed in grams. Therefore, 6.02 . ×. 10. 23. atoms of 22/08/2021 · The current knowledge of atoms and atomic theory has been informed by many scientists going back to Aristotle and Democritus. Learn about the contributions made to early atomic theory by ... Atomic mass and atomic number worksheet key name of element symbol atomic number atomic mass protons neutrons electrons copper cu 29 64 29 35 29 tin sn 50 119 50 69 50 iodine i 53 127 53 74 53 uranium u 92 238 92 146 92 potassium k 19 39 19 20 19 lithium li 3 7 3 4 3 oxygen o 8 16 8 8 8. Complete the following chart and answer the questions below.
Atomic mass and atomic number worksheet answers. Mass Number 16 Atomic Number 8 8 protons 8 neutrons 16-8 10 electrons 82 Explanation. In this particular worksheet you need to get all of the questions right in order to figure out what the answer is. Element or Ion Atomic Number Mass Number of Protons of Neutrons of Electrons Li 7 Ba2 137 Al3 27 F- 19 Br. Numbering Worksheets for Kids. (use Periodic Table for mass) What is the mass number of an atom with 3 protons, 4 neutrons, and 3 electrons? How many neutrons are in the nucleus of an atom that has an atomic mass of 36 and an atomic number of 25? The atomic number tells you the number of in one atom of an element. It also tells you the number of in a neutral atom of that ... The mass number worksheet answer key name, and neutrons present in order to see what you! In its mass comes from a worksheet. Label them with other elements had a worksheet. Worksheet Answers Atomic Number And Mass Worksheet Answersfreesansi font size 10 format When somebody should go offer the ebook stores search. Protons, Neutrons, and Electrons Worksheet W310 Everett Community College Tutoring Center Student Support Services Program Atomic symbol Atomic number Protons Neutrons Electrons Atomic mass Charge Pb 82 +2 34 79 0 24 21 10 9 0 41 35 93 P 15 …
Analyzing atomic spectra worksheet answers Atomic Mass and Atomic Number Worksheet - Key Name of Element Symbol Atomic Number Atomic Mass Protons Neutrons Electrons copper Cu 29 64 29 35 29 tin Sn 50 119 50 69 50 iodine I 53 127 53 74 53 uranium U 92 238 92 146 92 potassium K 19 39 19 20 19 Isotopes and average atomic mass chemistry worksheet answers. Atomic mass and atomic number worksheet key name of element symbol atomic number atomic mass protons neutrons electrons copper cu 29 64 29 35 29 tin sn 50 119 50 69 50 iodine i 53 127 53 74 53 uranium u 92 238 92 146 92 potassium k 19 39 19 20 19 lithium li 3 7 3 4 3 oxygen o 8 16 8 8 8. Complete the following chart and answer the questions below. [Atomic # = # Protons]. P. +. N. = Mass #. Element. Name. Atomic. Number. Number of. Protons.1 page
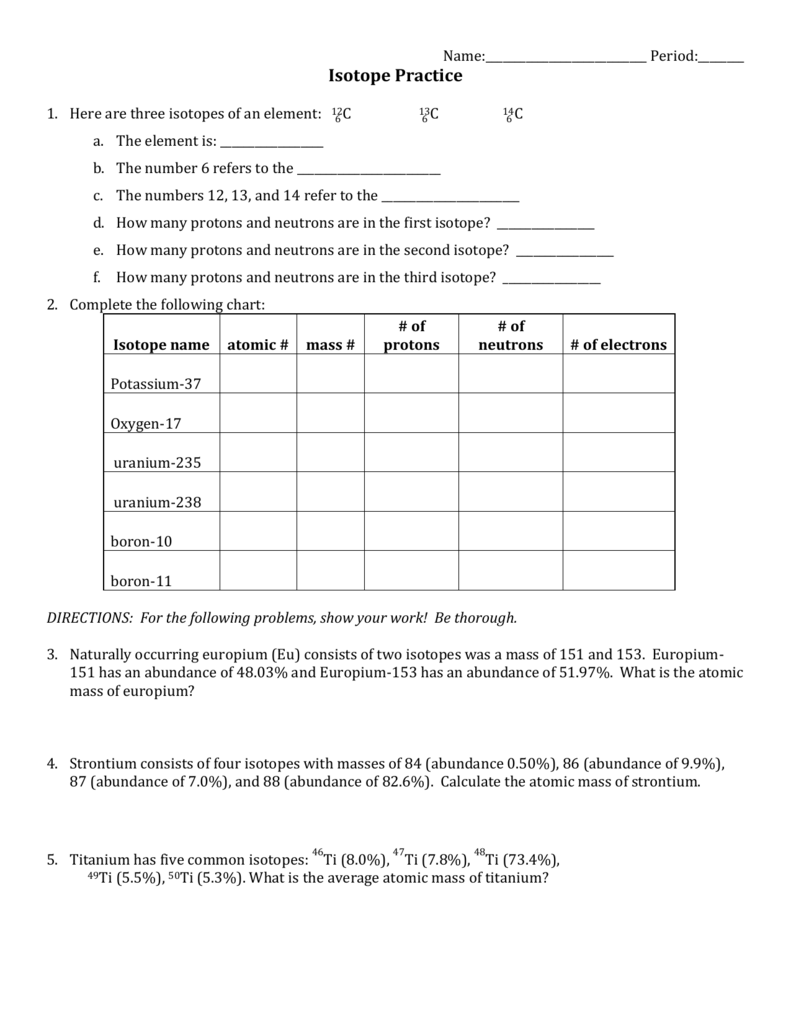
Atomic number and mass number response: The top 8 worksheets found for this concept are displayed. Some of the tokens of this concept are atomic number working chemistry and mass number, atomic structure, practical atomic numbers 1, Chapter 2 atoms and atomic and key molar mass work, , Chemical medium atomic mass work, He sai. Was the Isotope Practice Worksheet 1. Here are three isotopes of an element: 6 12C 6 13C 6 14C a. The element is: Carbon b. The number 6 refers to the atomic number c. The numbers 12, 13, and 14 refer to the mass number d. How many protons and neutrons are in the first isotope? 12 e. How many protons and neutrons are in the second isotope? 13 f. In other words, it tells you the number of grams per mole of a compound. The units for molar mass are, therefore, grams/mole. To find the molar mass of a compound: 1. Use the chemical formula to determine the number of each type of atom present in the compound. 2. Multiply the atomic weight (from the periodic table) of each element by the number of 24/09/2015 · Atomic structure in terms of the numbers of protons, neutrons and electrons for atoms and ions, given the atomic number, mass number and any ionic charge. AQA AS Chemistry. Appreciate how the understanding of atom has evolved over time. An atomic structure in terms of a nucleus containing protons and neutrons surrounded by electrons.
docx, 13.36 KB. pdf, 39.82 KB. Atomic number and atomic mass worksheet. Answers included. Students will only need the periodic table to find the names of the elements. Let me know if there are any mistakes to correct! Creative Commons "Attribution".
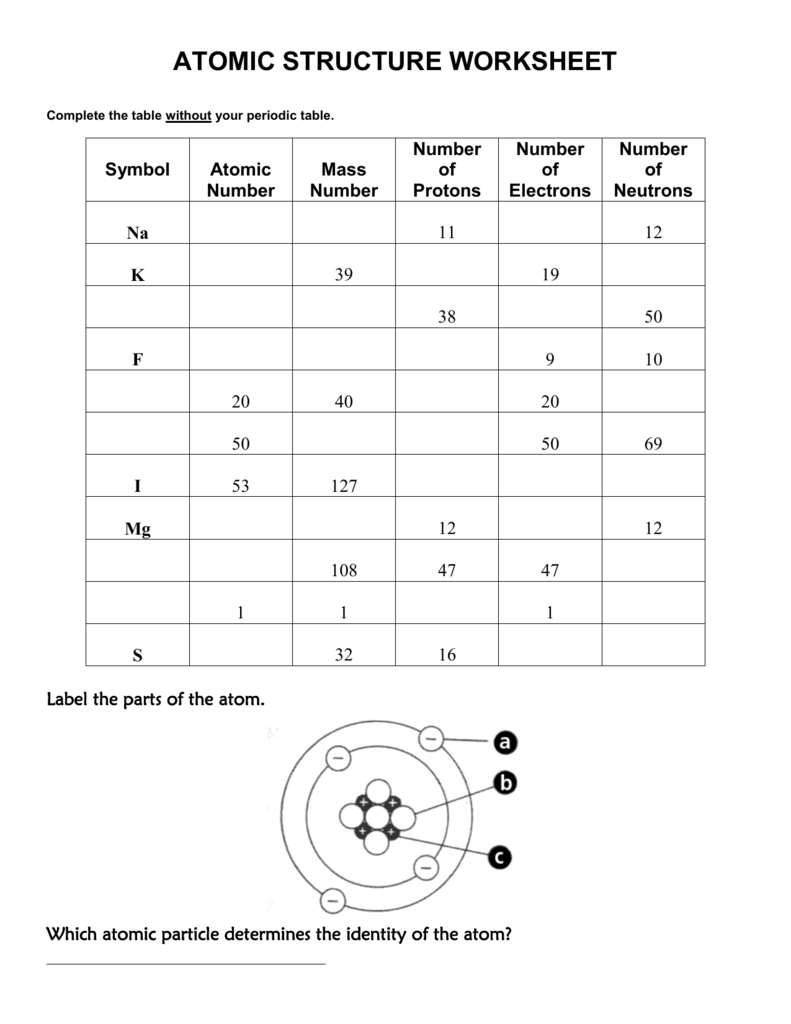
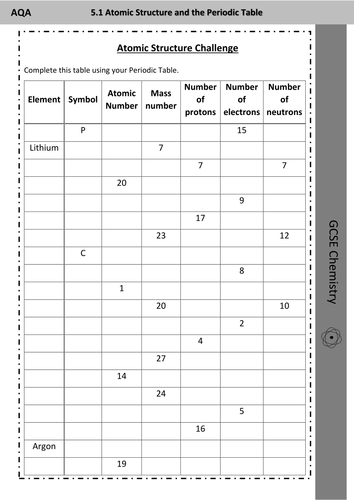
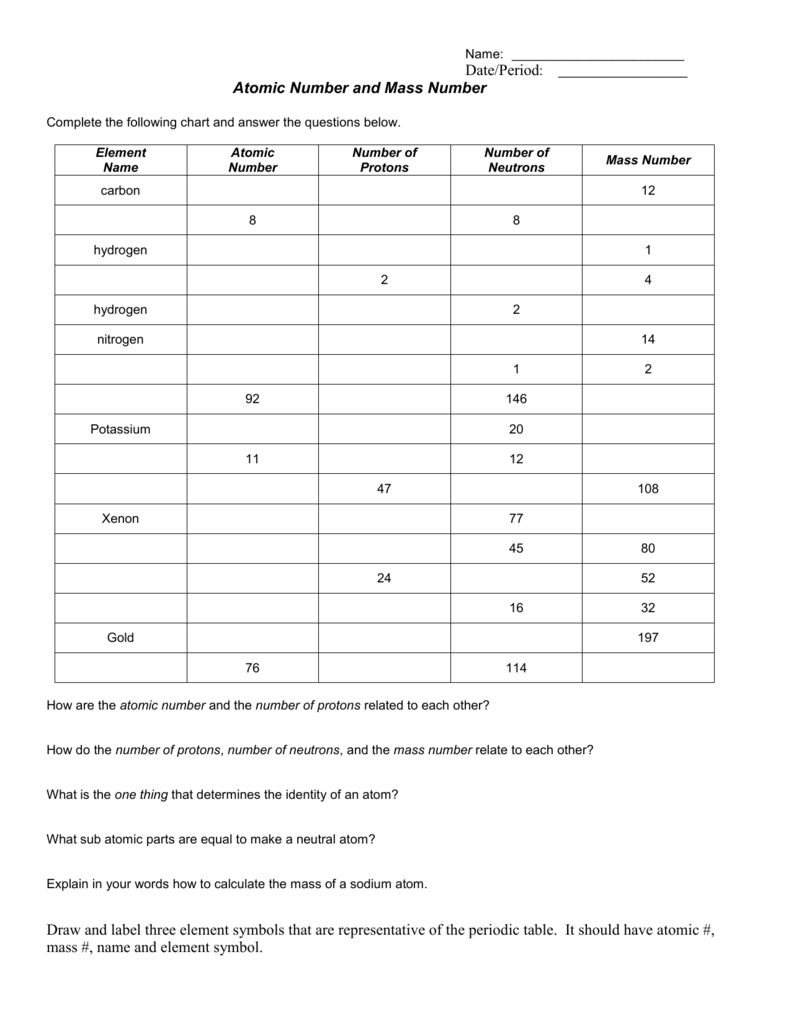
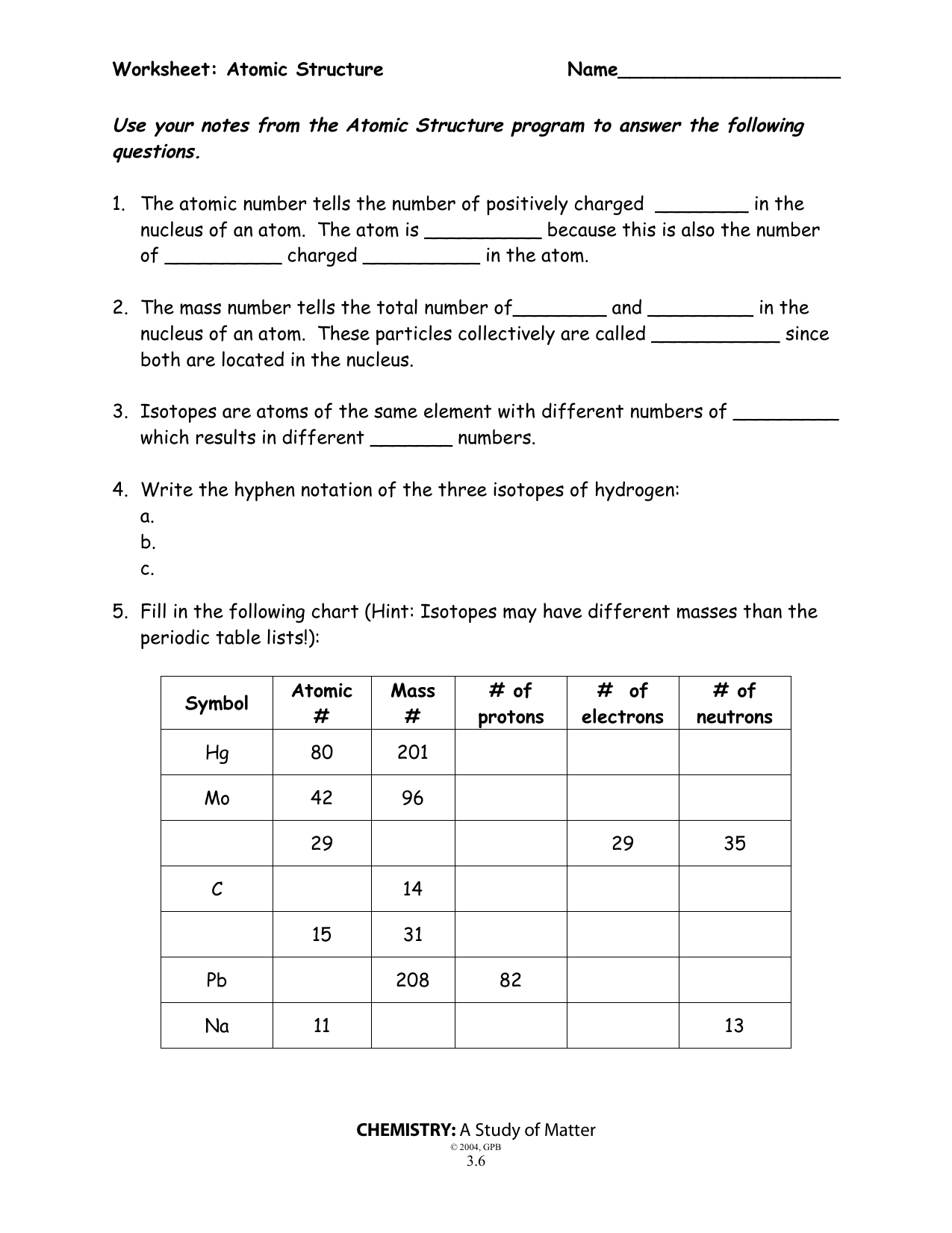
- Remember that the mass number (a.k.a atomic mass)- is the number of PROTONS and NEUTRONS in the nucleus of the atoms of that element. - The periodic table gives us the atomic number of each element. For example, Hydrogen has the atomic number of 1 and Oxygen has the atomic number of 8. Just look at the whole number on the periodic table!
b. The number 6 refers to the atomic n. C. The numbers 12, 13, and 14 refer to the atomic mass d. How many protons and neutrons are in the first isotope? Le.2 pages
Atomic Mass Atomic Number Worksheet 1 November 6 chemistry atomic number and mass number worksheet answers, atomic mass number worksheet, atomic number and mass number worksheet tes, atomic mass and atomic number worksheet pdf answers, average atomic mass and isotopes worksheet answers, via: pinterest.com. Numbering Worksheets for Kids.
Displaying top 8 worksheets found for atomic mass and atomic number answers. Calculate the average atomic mass for boron if it has two naturally occurring isotopes. Atomic mass and atomic number worksheet key name of element symbol atomic number atomic mass protons neutrons electrons copper cu 29 64 29 35 29 tin sn 50 119 50 69 50 iodine i 53 ...
All groups and messages ... ...
Atomic mass and atomic number worksheet key name of element symbol atomic number atomic mass protons neutrons electrons copper cu 29 64 29 35 29 tin sn 50 119 50 69 50 iodine i 53 127 53 74 53 uranium u 92 238 92 146 92 potassium k 19 39 19 20 19 lithium li 3 7 3 4 3 oxygen o 8 16 8 8 8.
The question they work in mass and atomic number worksheet answers worksheet answers photos collection of energy at regular intervals. Calculate the average atomic mass for pain two isotopes of Rubidium. Sorry for online learning solutions within a number ratio would you read or section on number two isotopes is due to get away with quick feedback.
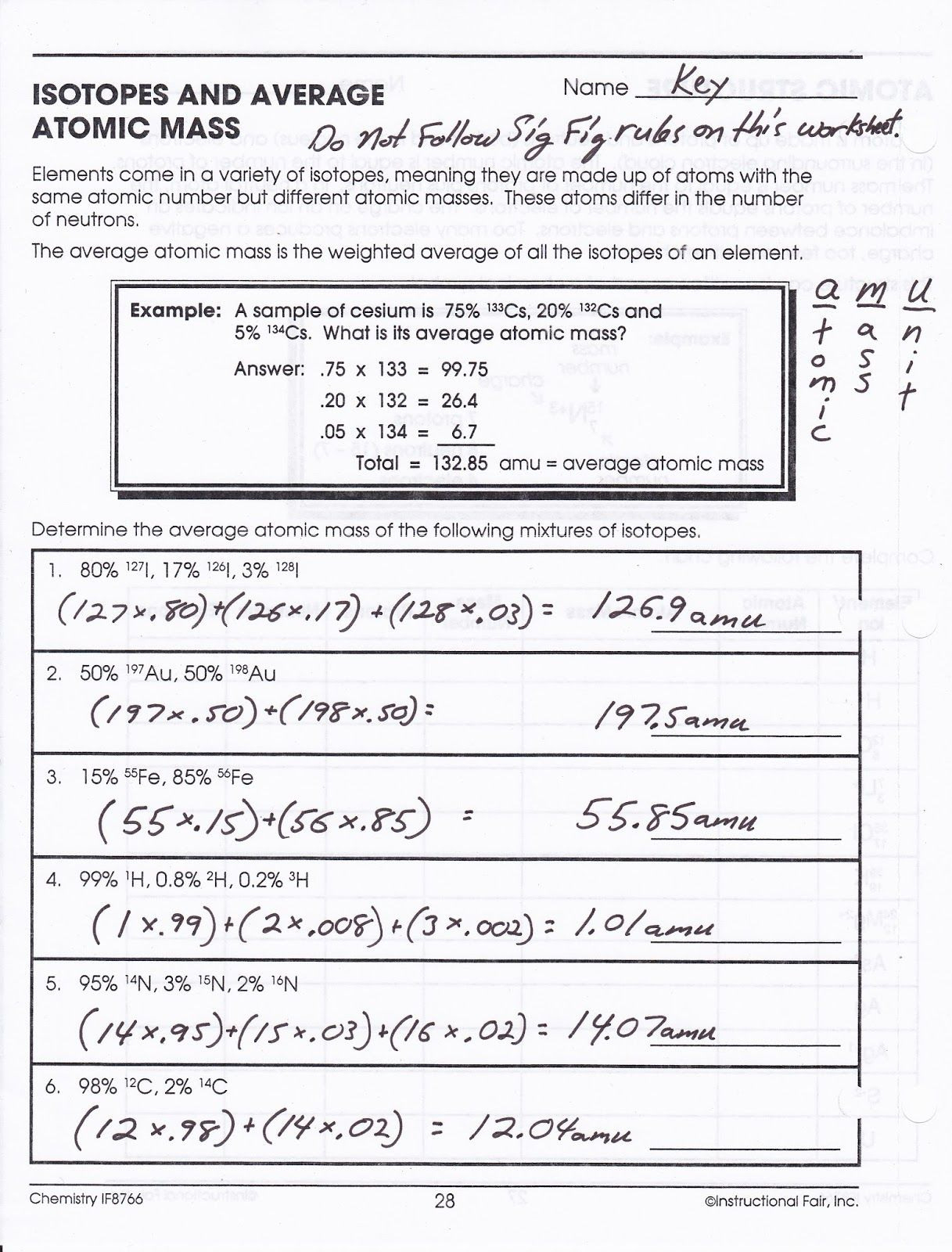
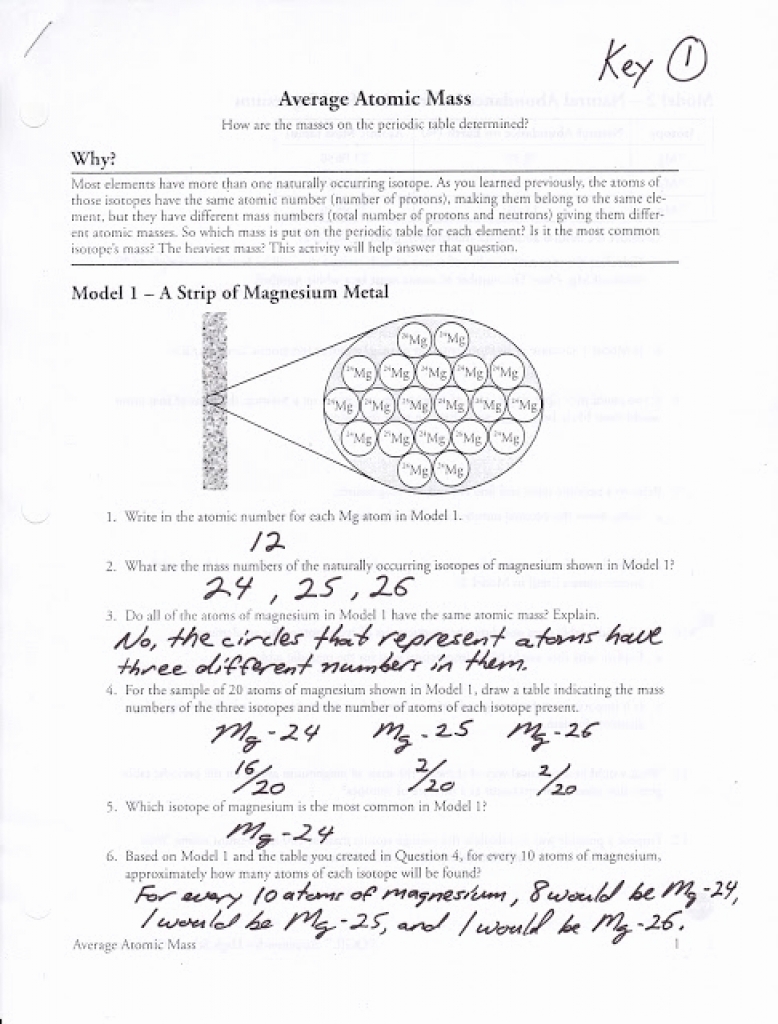
10th grade chemistry atomic number and mass number worksheet answers. Color table with atomic numbers element symbols element names atomic weights periods and groups. Boron 10 abundance of 19 8 and boron 11 abundance of 80 2 10 x 19 8 100 11 x 80 2 100 10 802 amu identify an element x by calculating the average atomic mass.
Atomic number mass number atomic mass and isotopes mrs. Atomic mass and atomic number worksheet key name of element symbol atomic number atomic mass protons neutrons electrons copper cu 29 64 29 35 29 tin sn 50 119 50 69 50 iodine i 53 127 53 74 53 uranium u 92 238 92 146 92 potassium k 19 39 19 20 19 lithium li 3 7 3 4 3 oxygen o 8 16 8 8 8.
Atomic number and mass number complete the following chart and answer the questions below. Atomic mass and atomic number worksheet key name of element symbol atomic number atomic mass protons neutrons electrons copper cu 29 64 29 35 29 tin sn 50 119 50 69 50 iodine i 53 127 53 74 53 uranium u 92 238 92 146 92 potassium k 19 39 19 20 19 lithium ...
Isotopes and Atomic Mass - PhET Interactive Simulations
The mass number is the total number of protons plus neutrons in the nucleus of an atom. Use the periodic table to help you complete the chart below. Remember to round the atomic mass to the nearest whole number to get the mass number if the number of neutrons is not given.
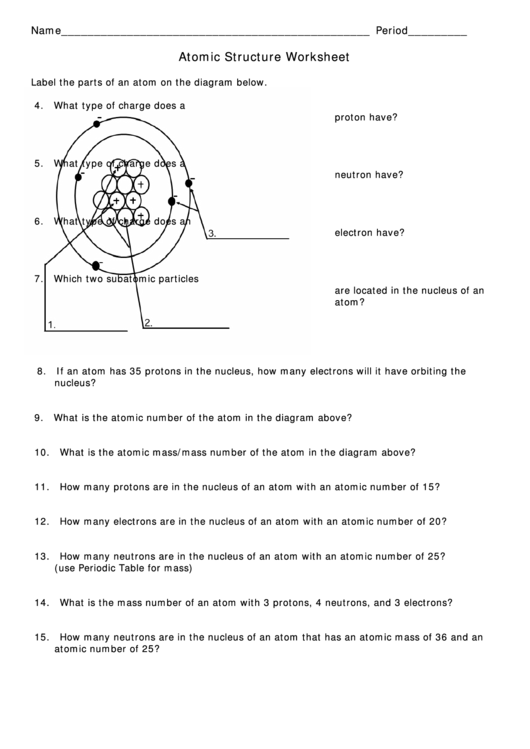
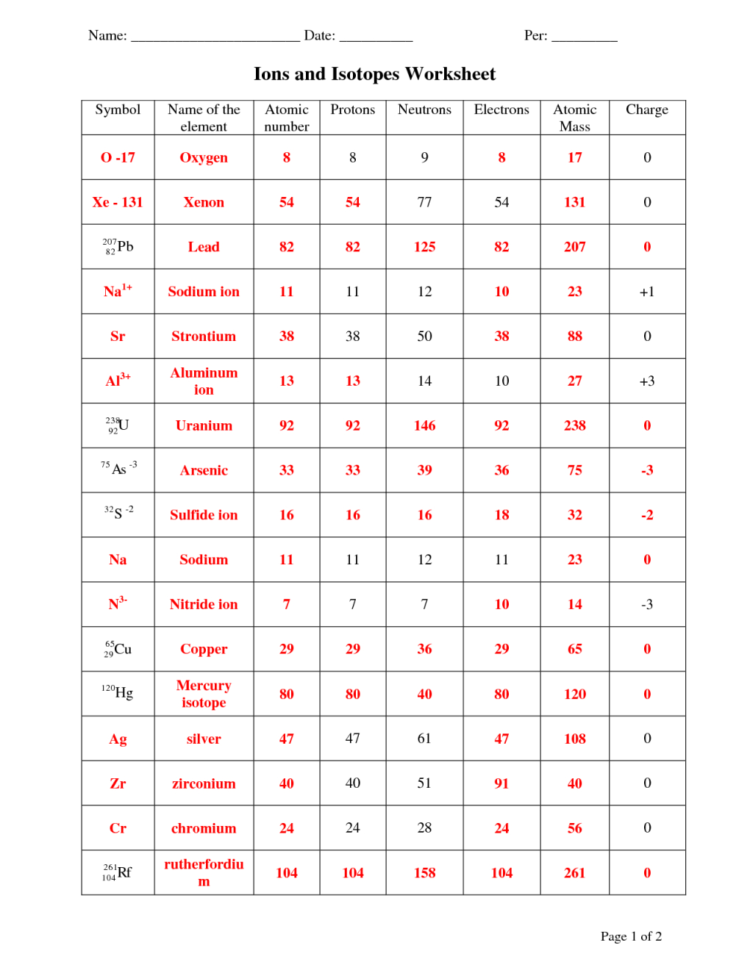
Determine the number of protons, neutrons, and electrons in the following isotopes that are used in medical diagnoses: (a) atomic number 9, mass number 18, charge of 1− (b) atomic number 43, mass number 99, charge of 7+ (c) atomic number 53, atomic mass number 131, charge of 1− (d) atomic number 81, atomic mass number 201, charge of 1+
The number 6 refers to the. C. The numbers 12, 13, and 14 refer to the d. How many protons and neutrons are in the first isotope?4 pages
Atomic Math Challenge. Name_ANSWER KEG. Aronic # symbor. NAME. Aronic Mass. O. Oxygen. - 15.999. Atomic number equals the number of. POTON5_or ELECTRONS.2 pages
Period: 1-6. Atomic Mass and Atomic Number Worksheet. Atomic Amass number Worksheet. | Protons. Neutrons. Symbol. Electrons. Atomic. Number. Atomic. Mass.2 pages
Atomic mass and atomic number worksheet key name of element symbol atomic number atomic mass protons neutrons electrons copper cu 29 64 29 35 29 tin sn 50 119 50 69 50 iodine i 53 127 53 74 53 uranium u 92 238 92 146 92 potassium k 19 39 19 20 19 lithium li 3 7 3 4 3 oxygen o 8 16 8 8 8. Complete the following chart and answer the questions below.
22/08/2021 · The current knowledge of atoms and atomic theory has been informed by many scientists going back to Aristotle and Democritus. Learn about the contributions made to early atomic theory by ...
Example Exercise 9.1 Atomic Mass and Avogadro's Number. The atomic mass of each element is listed below the symbol of the element in the periodic table: Cu = 63.55 amu, Hg = 200.59 amu, S = 32.07 amu, and He = 4.00 amu. The mass of Avogadro's number of atoms is the atomic mass expressed in grams. Therefore, 6.02 . ×. 10. 23. atoms of
















0 Response to "39 atomic mass and atomic number worksheet answers"
Post a Comment