38 mole to mole worksheet
Worksheet for Basic Stoichiometry. Part 1: Mole ←→ Mass Conversions. Convert the following number of moles of chemical into its corresponding mass in grams. 1. 0.436 moles of ammonium chloride. 2. 2.360 moles of lead (II) oxide. 3. 0.031 moles of aluminum iodide. 4. 1.077 moles of magnesium phosphate. 5. 0.50 moles of calcium nitrate Molar Enthalpy Worksheet - side A Q=mΔH (Q= energy or heat in KJ, m=moles, ΔH=molar enthalpy ) A change in enthalpy (ΔH) is a measurement of e nergy transfer in the form of heat . M olar enthalpy is the enthalpy change per mole of a substance involved in a transformation.
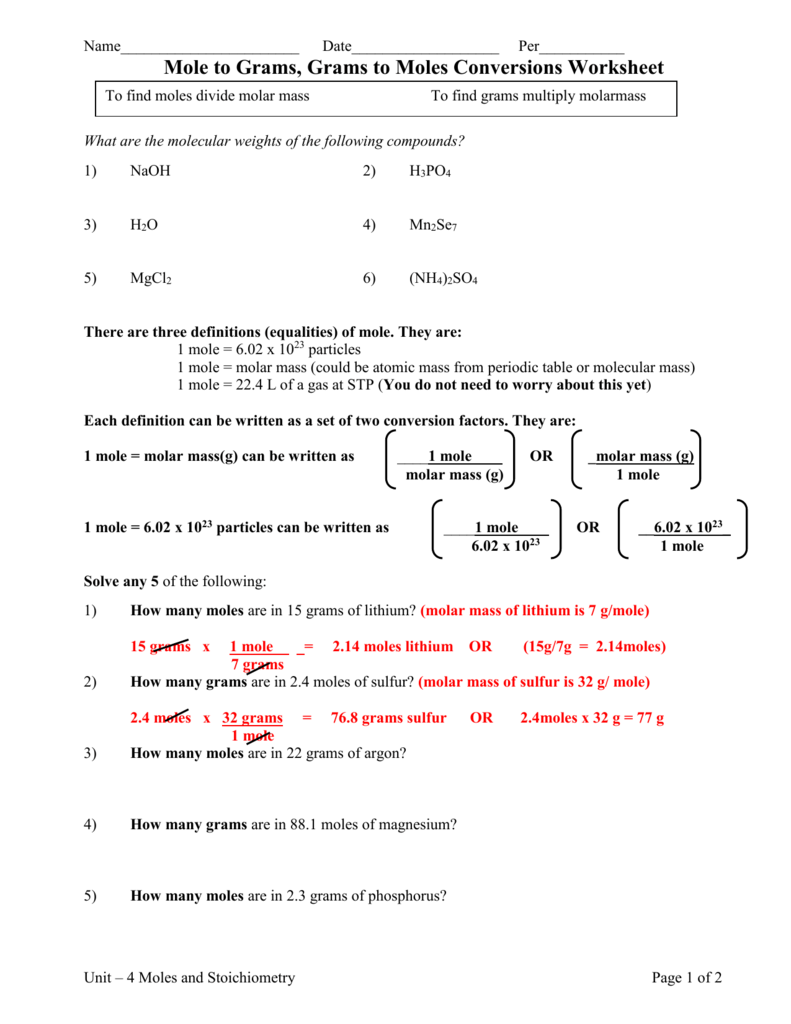
Mole to Grams, Grams to Moles Conversions Worksheet What are the molecular weights of the following compounds? (all masses must be to nearest hundredth) 1) NaOH 2) H 3 PO 4 3) H 2 O 4) Mn 2 Se 7 5) MgCl 2 6) (NH 4) 2 SO 4 There are three definitions (equalities) of mole. They are: 1 mole = 6.02 x 1023 particles
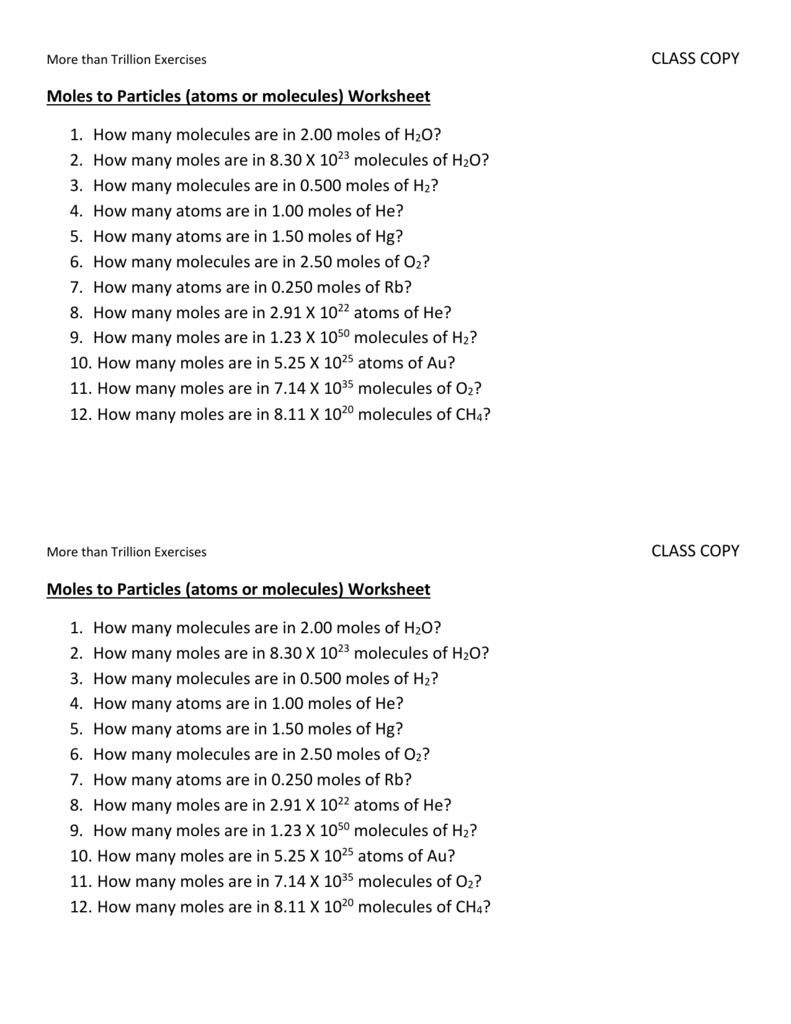
Mole to mole worksheet
Jun 30, 2021 · While the mole ratio is ever-present in all stoichiometry calculations, amounts of substances in the laboratory are most often measured by mass. Therefore, we need to use mole-mass calculations in combination with mole ratios to solve several different types of mass-based stoichiometry problems. holds for each component of the solution, calculate the mole fraction of benzene in the vapor. ( molar mass of benzene= 78.0 g/mole and toluene = 92.0 g/mole.) Answer= 0.87 6. The freezing point of a glucose solution ( C6H12O6;molar mass= 180.0 g/mole) is - 10.3 ° C . The density of the solution is 1.50 g/ml. Mole Calculation Practice Worksheet Solutions Answer the following questions: 1) How many moles are in 25.0 grams of water? 1.39 moles 1 mole H 2O = 18.0 g H 2O 25 g H 2O 1 mol H 2O 18.0 g H 2O 2) How many grams are in 4.500 moles of Li 2O? 134.6 grams
Mole to mole worksheet. Mole Calculation Worksheet W 340 Everett Community College Tutoring Center Student Support Services Program 1) How many moles are in 40.0 grams of water? 2) How many grams are in 3.7 moles of Na 2 O? 3) How many atoms are in 14 moles of cadmium? Worksheet – Mole Conversions Name: Show all work utilizing dimensional analysis wherever possible. Include all units and account for significant figures. A. What is the molar mass of: (make sure to show clearly defined and complete work here) 1. H2 1 . mol H x 2.02 g H = 2.02 g H2 1 mol H2. 2. Ba(OH)2 Use dimensional analysis to convert between the mass, number of moles and number of particles of a substance. 10.2-3. Directions: Show ALL of your work. Make sure to include units!!!! Mole-Particle Conversions ... Mole Conversions Worksheet Last modified by: Anoka-Hennepin Company: Mole Fraction/Molality Worksheet Name: Date: 1. A solution is prepared by mixing 100.0 g of water, H2O, and 100.0 g of ethanol, C2H5OH. Determine the mole fractions of each substance. 2. The molality of an aqueous solution of sugar (C12H22O11) is 1.62m. Calculate the mole fractions of sugar and water. 3.
STOICHIOMETRY WORKSHEET (MOLE-MOLE) I. Magnesium reacts with hydrochloric acid according to the following balanced chemical equation: Mg (s) + 2 HCI (aq) MgC12 (aq) + Ha (g) If two moles of h drochloric acid react with excess magnesium, how many moles of hy rogen gas will be produced? 2 mole Hd I mole H 2 H2 HCI 2. Mole Calculation Worksheet – Answer Key 1) How many moles are in 15 grams of lithium? 0.46 moles 2) How many grams are in 2.4 moles of sulfur? 77.0 grams 3) How many moles are in 22 grams of argon? 0.55 moles 4) How many grams are in 88.1 moles of magnesium? 2141 grams 5) How many moles are in 2.3 grams of phosphorus? 0.074 moles Sep 24, 2021 · Collectively, these conversions are called mole-mass calculations. As an example, consider the balanced chemical equation \[Fe_2O_3 + 3SO_3 \rightarrow Fe_2(SO_4)_3\] If we have 3.59 mol of Fe 2 O 3, how many grams of SO 3 can react with it? Using the mole-mass calculation sequence, we can determine the required mass of SO 3 in two steps. MOLE WORKSHEET #2 Make the following conversions using unit analysis. Use a separate piece of paper, show all work, and circle your final answer. (Attach this sheet to your work). Set A: One Step Problems: Convert to moles: Convert to mass in grams: 10.0 moles Na 11. 12. 2.20 moles Sn 13. 5.00 moles Ag 14. 3.0 x 104 moles Au 15. 1.00 x 10-7 moles B
Nov 17, 2021 · A mole is a chemical counting unit, such that 1 mole = 6.022*10 23 particles. Stoichiometry also requires the use of balanced equations. Stoichiometry also requires the use of balanced equations. Mole Calculation Practice Worksheet Solutions Answer the following questions: 1) How many moles are in 25.0 grams of water? 1.39 moles 1 mole H 2O = 18.0 g H 2O 25 g H 2O 1 mol H 2O 18.0 g H 2O 2) How many grams are in 4.500 moles of Li 2O? 134.6 grams holds for each component of the solution, calculate the mole fraction of benzene in the vapor. ( molar mass of benzene= 78.0 g/mole and toluene = 92.0 g/mole.) Answer= 0.87 6. The freezing point of a glucose solution ( C6H12O6;molar mass= 180.0 g/mole) is - 10.3 ° C . The density of the solution is 1.50 g/ml. Jun 30, 2021 · While the mole ratio is ever-present in all stoichiometry calculations, amounts of substances in the laboratory are most often measured by mass. Therefore, we need to use mole-mass calculations in combination with mole ratios to solve several different types of mass-based stoichiometry problems.




























0 Response to "38 mole to mole worksheet"
Post a Comment