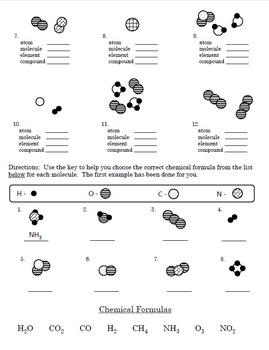
38 atoms and bonding worksheet
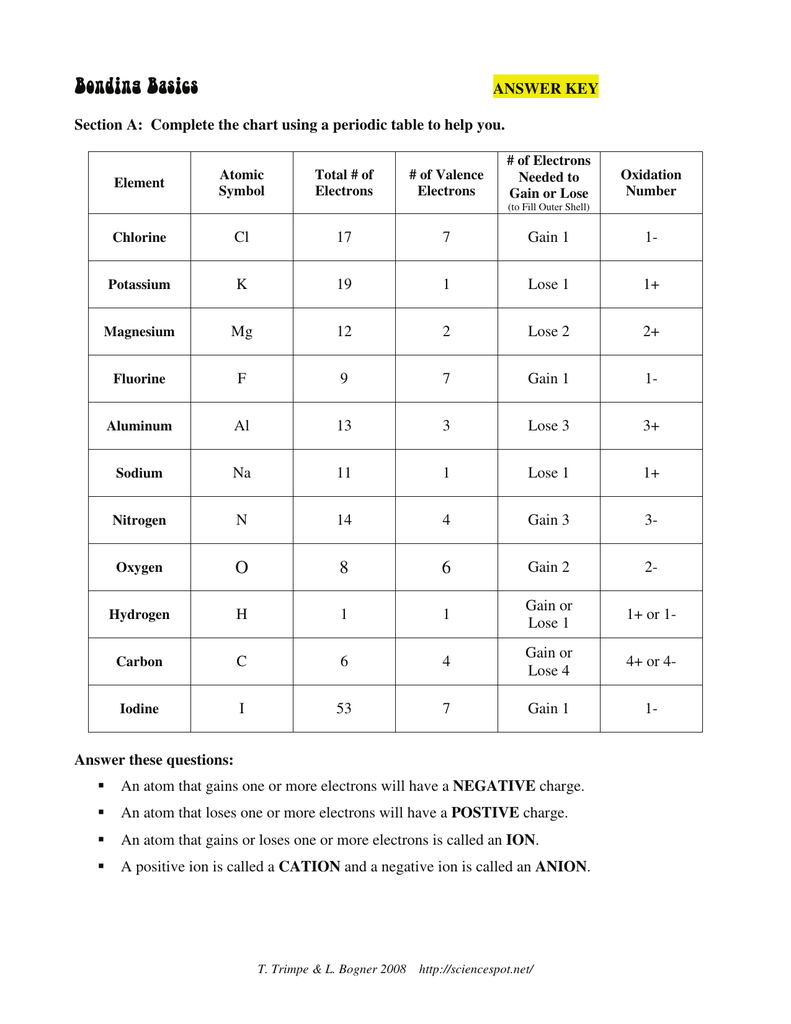
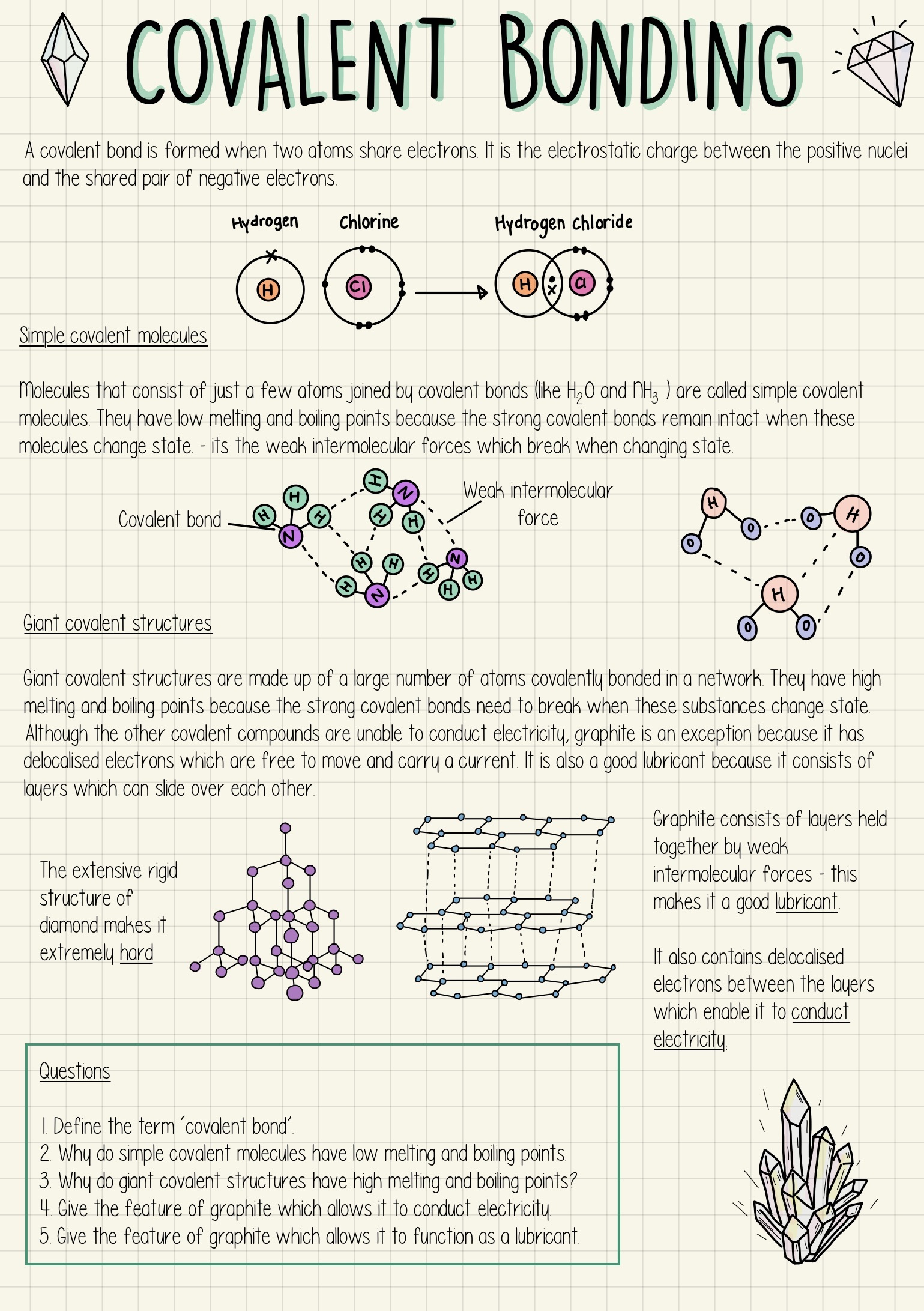
Atoms with four or more valence electrons form as many bonds as electrons needed to fill the s and p levels of their valence shells to reach a stable octet. Carbon has four valence electrons (2s2 2p2), forming four bonds (CH 4). Development of Chemical Bonding Theory valence e- IMF – Intermolecular Forces Worksheet Indicate the strongest IMF holding together thousands of molecules of the following. Then indicate what type of bonding is holding the atoms together in one molecule of the following. NOTE – if the molecule is an ionic compound, then there is no IMF, the ions are all held together by ionic bonds. IMF ...
Created Date: 12/5/2018 11:51:32 PM

Atoms and bonding worksheet
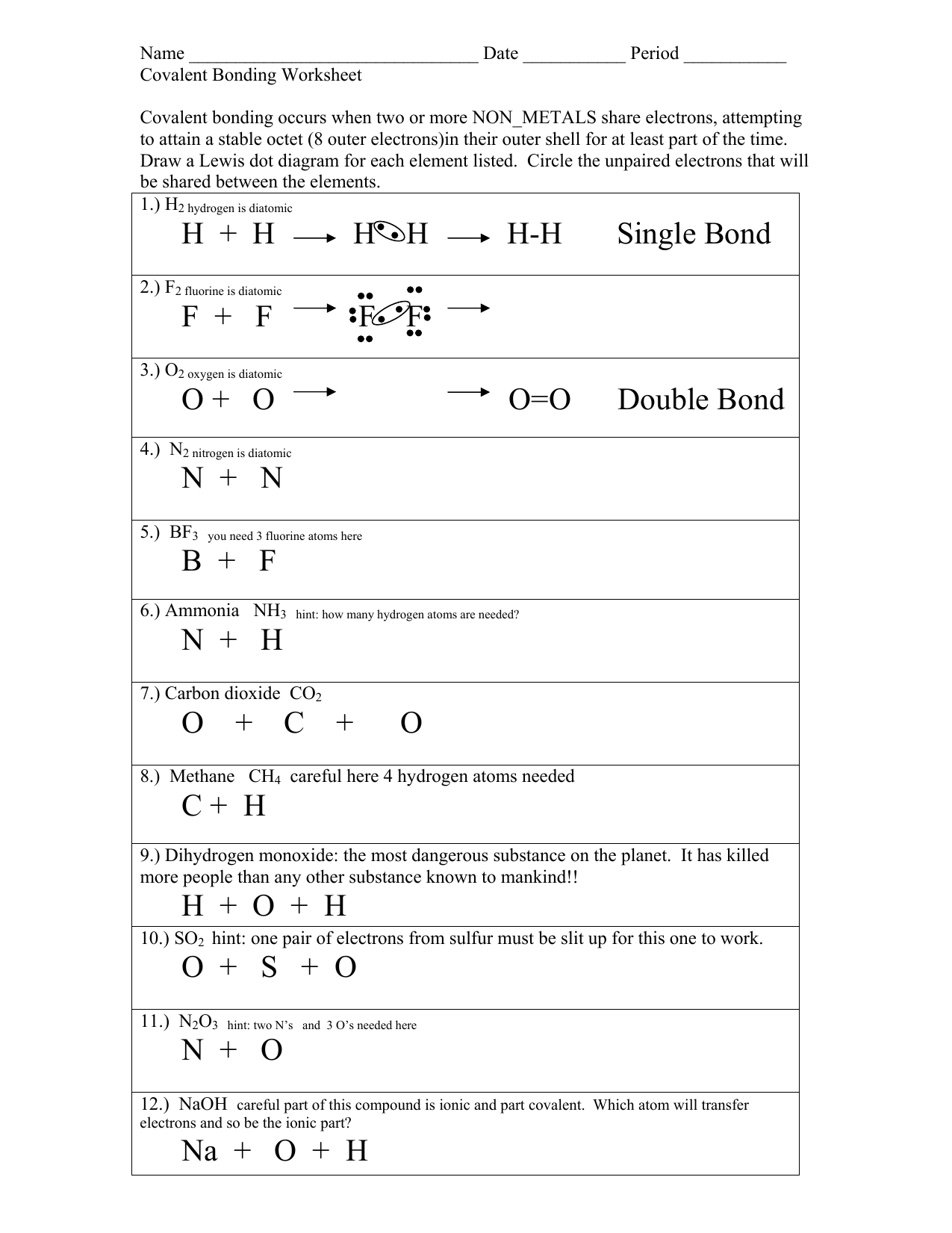
The electrons are shared between the atoms, dividing their time between them to “fill” the outer shell of each. This sharing is a lower energy state for all of the atoms involved than if they existed without their outer shells filled. There are two types of covalent bonds: polar and nonpolar. Explore molecule shapes by building molecules in 3D! How does molecule shape change with different numbers of bonds and electron pairs? Find out by adding single, double or triple bonds and lone pairs to the central atom. Metallic Bonding: The Electron-Sea ... It describes how the orbital shapes combine when atoms combine into molecules. ... This worksheet and quiz will let you practice the following skills:
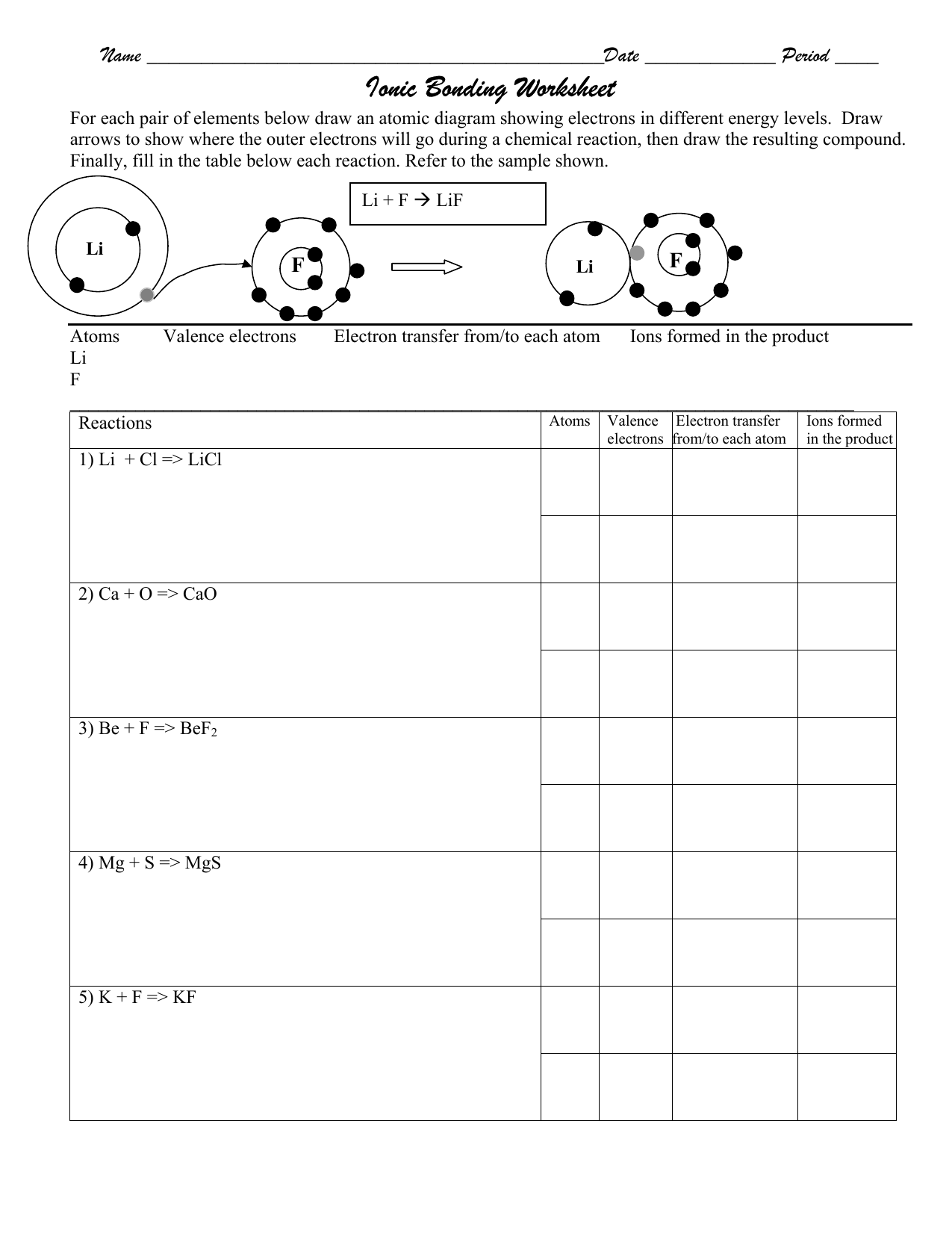
Atoms and bonding worksheet. Essential Question: How is ionic bonding different from covalent bonding? Comparison of Properties of Ionic and Covalent Compounds Because of the nature of ionic and covalent bonds, the materials produced by those bonds tend to have quite different macroscopic properties. The atoms of covalent materials are bound tightly to each other in stable Metallic Bonding: The Electron-Sea ... It describes how the orbital shapes combine when atoms combine into molecules. ... This worksheet and quiz will let you practice the following skills: Explore molecule shapes by building molecules in 3D! How does molecule shape change with different numbers of bonds and electron pairs? Find out by adding single, double or triple bonds and lone pairs to the central atom. The electrons are shared between the atoms, dividing their time between them to “fill” the outer shell of each. This sharing is a lower energy state for all of the atoms involved than if they existed without their outer shells filled. There are two types of covalent bonds: polar and nonpolar.
























0 Response to "38 atoms and bonding worksheet"
Post a Comment