41 chemistry worksheet wavelength frequency and energy
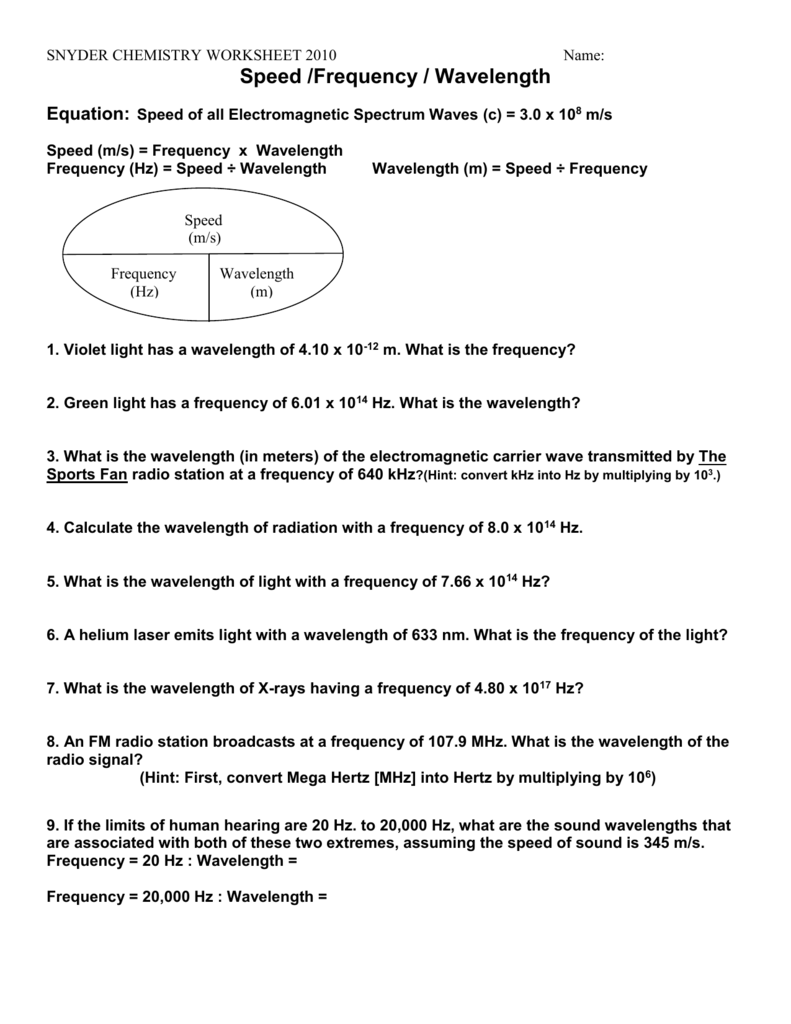
Nov 03, 2011 · Calculate the wavelength of radiation with a frequency of 8.0 x 1014 Hz. w=c/f =3.00x108/8.0*1014 =0.375x10(8-14) m =0.375x10-6 m =3.75X10-7 m. Calculating Energy & Frequency of EM radiation. Defining variables. Frequency (Hz) = f. Energy (Joules) = J. Planck’s constant = h = 6.626 x 10-34 Joules*s. Deriving equations
Chemistry Worksheet – Wavelength, frequency, & energy of electromagnetic waves. ANSWER KEY . Show ALL equations, work, units, and significant figures in performing the following calculations. Identify the type of radiation in each problem. (Use your electromagnetic spectrum) C = λν E = hν. C = 3.00 x 108 m/s h = 6.626 2 x 10-34 J-s (or J/Hz) eV = 1.602 x 10 …
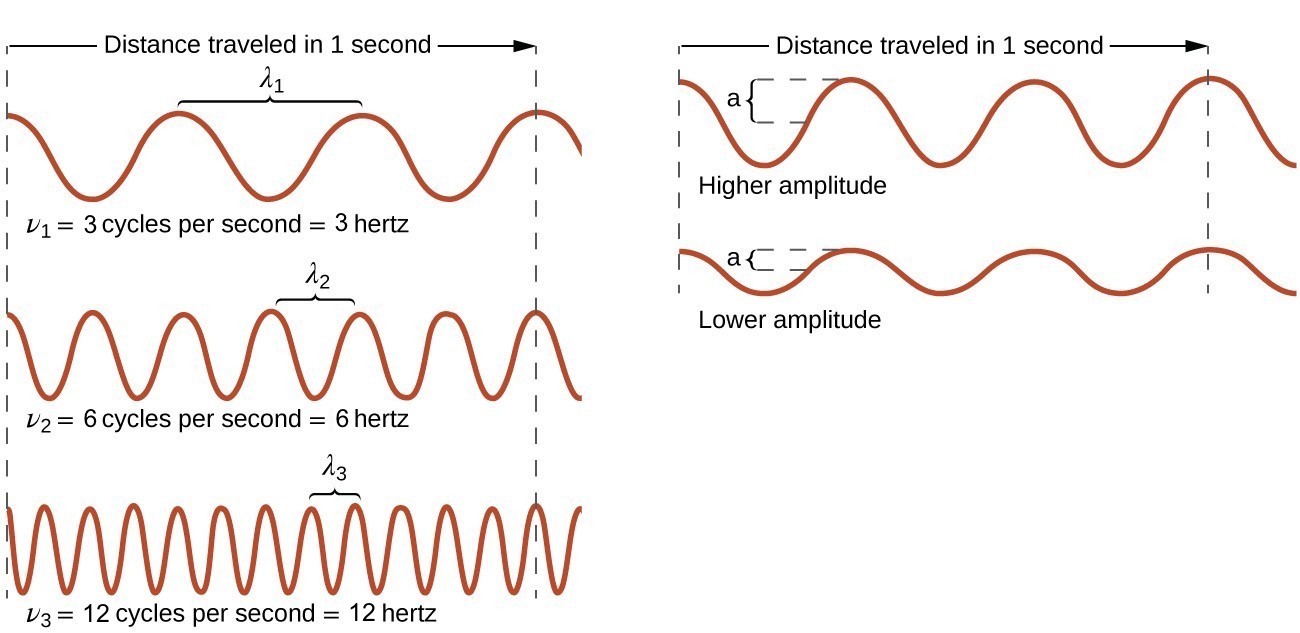
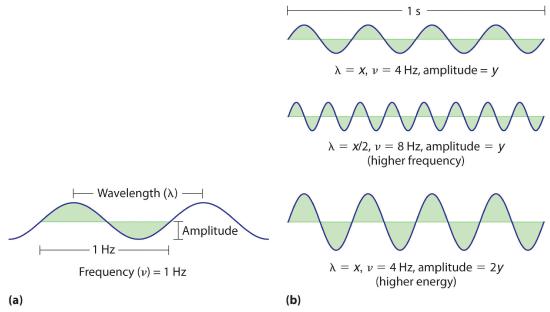
27.11.2021 · Energy Content: Amplitude and Frequency. The amplitude of a wave is how the wave is measured from the rest position or midline to the top of a crest or bottom of a trough, measured in meters. A ...
Chemistry worksheet wavelength frequency and energy
27.08.2021 · Wave parameters refer to the different ways waves are measured. Examine how waves are characterized by wavelength, amplitude, period, frequency, and/or speed.
Photon Energy and deBroglie Wavelength worksheet 1) The alpha line in the Balmer series of the hydrogen spectrum consists of light having a wavelength of 6. I failed the test. Example: is Is 2s 2P . Ground state electron configurations can be predicted by a strict set of rules known as the Aufbau principle (“aufbau”means filling up). 1 & 19. Some pigments, including chlorophyll, can …
Chapter 7 Wavelength, Frequency, Speed & Energy Practice Worksheet Formulas and Constants: f = c/λ E = hf c = speed of light (3.0 x 108 m/s) λ = wavelength f = frequency E = energy h = Planck’s constant (6.6262 x 10-34 J•s) 1. 14Calculate the λ given the frequency of radiation is 5.10 x 10 hz. 2.
Chemistry worksheet wavelength frequency and energy.
22.11.2019 · Alphabetical Index of Chemistry Problem Types . Included in this list are printable pdf chemistry worksheets so you can practice problems and then check your answers. You may also browse chemistry problems according to the type of problem.
Department of Chemistry University of Texas at Austin Tera 1012 Giga 109 Mega 106 Kilo 103 Hecto 102 Deca 101 ☐ deci 10-1 centi 10-2 milli 10-3! micro 10-6 nano 10-9 pico 10-12 fempto 10-19 More Practice: Energy, Frequency, Wavelength and the Photoelectric Effect. There are two equations you should know: E = h! and c =!!" !=!!!!!∴!!!=!!! E = energy (J) = wavelength (m) ! = …
14.02.2019 · Some of the worksheets below are Waves Worksheets Middle School in PDF, label parts of a wave and describe some of the properties and behavior of waves, wave types, wave speed equation practice problems, understand crests, troughs, wavelength, amplitude, …
The temperature outside is 32 C. T Evaporation is a physical change. website builder. adjusting to reality free chemistry 12 honors chem unit 2 atomic structure electron This''HONORS CHEMISTRY UNIT 3 WORKSHEET 1 ANSWERS DECEMBER 20TH, 2019 - UNIT 3 WORKSHEET 3 QUANTITATIVE ENERGY PROBLEMS PART 1 MODELING CHEMISTRY 1 …
Determining the Harmonic Frequencies. Consider an 80-cm long guitar string that has a fundamental frequency (1st harmonic) of 400 Hz. For the first harmonic, the wavelength of the wave pattern would be two times the length of the string (see table above); thus, the wavelength is 160 cm or 1.60 m.The speed of the standing wave can now be determined from the …
Chemistry Worksheet – Wavelength, frequency, & energy of electromagnetic waves. ANSWER KEY Show ALL equations, work, units, and significant figures in performing the following calculations. Identify the type of radiation in each problem. (Use your electromagnetic spectrum) C = λν E = hν C = 3.00 x 108 m/s h = 6.626 2 x 10-34 J-s (or J/Hz)
Chemistry Worksheet and Answers Wavelength, frequency, & energy of electromagnetic waves. C = λν E = hν C = 3.00 x 108 m/s h = 6.626 2 x 10-34 J-s (or J/Hz) 1. What is the wavelength of a wave having a frequency of 3.76 x 1014 s-1? 2. What is the frequency of a 6.9 x 10-13 m wave? 3. What is the wavelength of a 2.99 s-1 wave? 4.
The above example illustrates how to use the wave equation to solve mathematical problems. It also illustrates the principle that wave speed is dependent upon medium properties and independent of wave properties. Even though the wave speed is calculated by multiplying wavelength by frequency, an alteration in wavelength does not affect wave speed.




























0 Response to "41 chemistry worksheet wavelength frequency and energy"
Post a Comment