39 bonding and molecular structure worksheet
Chapter 7 Worksheet Spring 2007 page 4 of 5 Formula ABE formula Number of e-domains on central atom # e- domains/ # non-bonding domains on central atom Electron-Domain Geometry Molecular Geometry (name) Bond angle(s) on central atom CO O 3 CO 3 2-SO 2 PF 5 PCl 5 SF 6 TeF 6 11) a. Identify the molecules in the table above that are polar. b.
Currently I'm in a unit on covalent bonding and we're working with lewis structures right now. We have a worksheet where we're given a molecule and told to fill out the molecular geometry, resonance, bond angles, etc. The thing that's tripping me up is isomers. How do I know when a molecule has isomers and when it doesn't? Does a molecule have isomers when it has resonance?
Dear All, We have vast range of test banks, solution manuals and Ebooks of all topics, If you need any solution manual, testbank for testbooks from the list below do contact us anytime, save your time and effort and let you definitely understand what you are studying and get an amazing marks as well. Contact us 24/7 : smtbportal @ gmail . com smtbportal(at)gmail(dot)com **Note:- Please do not reply to this message, send us email for any solution manual testbank Ebook inquiry at** **( smtb...
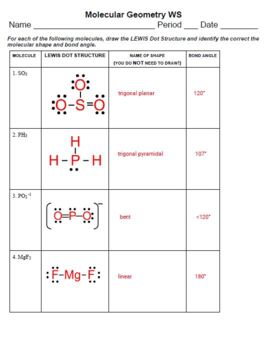
Bonding and molecular structure worksheet
Covalent Bonds and Molecular Structure 1) How are ionic bonds and covalent bonds different? Ionic bonds result from the transfer of electrons from one atom to another; Covalent bonds result from two atoms sharing electrons. 2) Describe the relationship between the length of a bond and the strength of that bond. Strength of a bond increases as the bond gets shorter (inverse relationship)
Rules: please make a study plan full of resources on all these topics: Chem final topics 1. Finding molecular/empirical formula from mass 2. Deducing amount of molecule produced from equation 3. Make balanced reaction from combustion, electrolytic decomposition, formation 4. Incomplete and complete combustion 5. Sodium metal and water reaction 6. Balancing 7. Bonding, polar covalent 8. Isotopes 9. Electron arrangement 10. Lewis structure 11. Bond angles 12. Atomic radii 13. Bond length 14. Mol...
Chemical Bonding And Molecular Structure Class 11 Chemistry Worksheet Pdf. Class 11 Chemistry students should refer to the following printable worksheet in Pdf for Chemical Bonding And Molecular Structure in standard 11. This test paper with questions and answers for Grade 11 Chemistry will be very useful for exams and help you to score good marks
Bonding and molecular structure worksheet.
6 • Bonding & Molecular Structure . LEWIS STRUCTURES . Indicate the # of VALENCE electrons for each species. Write the correct Lewis electron-dot structure for each. F o / K . Al # of valence e-'s = ~ # of valence e-'s = ----k:L # ofvalence e-'s = -L # of valence e -,s = 3 0 2 A, # of valence e-'s = ~ !!J . 0/0-/~) -I . r A1. 3 + # of valence e-'s= HF
X-posted from [/r/random_acts_of_amazon](http://www.reddit.com/r/Random_Acts_Of_Amazon) So, my biology teacher is awful. He barely teaches us anything and tests and homework are full of questions that nobody can find the answers to in the book, his lectures, online (we're allowed to look at other resources), etc. He literally tests us on stuff we've never learned. Basically, I need some emergency help on my homework because it's due tomorrow and I STILL can't figure out what the hell I'm doing ...
Chapters 6 and 7 Worksheet Spring 2013 page 1 of 5 Chapters 6 and 7 Practice Worksheet: Covalent Bonds and Molecular Structure 1) How are ionic bonds and covalent bonds different? 2) Describe the relationship between the length of a bond and the strength of that bond. 3) Identify the type(s) of bond(s) found in the following molecules: a. CCl 4
have a molecular geometry around each carbon atom that is trigonal planar (AX. 3), so all six atoms are in the same plane. The carbon atoms in C. 2. H. 6. have a molecular geometry that is tetrahedral (AX. 4), so the atoms are not all in the same plane. OR. The carbon-carbon double bond in C. 2. H. 4. results in a planar molecule whereas the carbon-carbon single bond in C

0 Response to "39 bonding and molecular structure worksheet"
Post a Comment